Abstract
Mammalian Glyco_18-domain–containing proteins include catalytically active chitinases and chitinase-like proteins with cytokine activity involved in host defense and Th2-type inflammatory reactions. Here, we describe a novel human Glyco_18-domain–containing protein, SI-CLP, as an interacting partner of the endocytic/sorting receptor stabilin-1. Similarly to the chitinase-like cytokines YKL-39, YKL-40, and YM1/2, SI-CLP lacks a chitin-binding domain and catalytic amino acids. Using a novel mAb 1C11, we demonstrated that SI-CLP is sorted into late endosomes and secretory lysosomes in human alternatively activated macrophages. The direct interaction of SI-CLP with stabilin-1, their colocalization in the trans-Golgi network, and the reduced sorting of SI-CLP into lysosomes in macrophages treated with stabilin-1 siRNA suggest that stabilin-1 is involved in intracellular sorting of SI-CLP. Expression of SI-CLP in macrophages was strongly up-regulated by the Th2 cytokine IL-4 and by dexamethasone. This effect was suppressed by IFNγ but not affected by IL-10. In contrast, expression of YKL-40 was induced by IFNγ and suppressed by dexamethasone. Macrophages treated with IL-4 secreted SI-CLP, while costimulation with dexamethasone blocked secretion and resulted in intracellular accumulation of SI-CLP. The 1C11 mAb detected SI-CLP in human bronchoalveolar lavage and peripheral-blood leukocytes (PBLs), and can be used to analyze the role of SI-CLP in human disorders.
Introduction
Macrophages are essential elements of innate immunity that orchestrate inflammatory reactions and immune tolerance as well as healing processes and tissue homeostasis. Macrophages perform these functions by the tightly regulated production of cytokines, enzymes, extracellular matrix (ECM) components, and other mediators. Alternatively, macrophages may also bind and internalize these molecules, thereby contributing to their inactivation and degradation. The high specificity and flexibility of this clearance function are based on the expression of multiple endocytic receptors by macrophages.1 The molecular patterns of macrophage secretory and clearance functions are regulated by the activation status and the polarization of the macrophages involved.2-4 Besides the classic, constitutively operating ER/Golgi secretory pathway, which is regulated primarily on the level of gene expression, macrophages use nonclassic5 and lysosomal secretory pathways.6-8 The lysosomal secretion route is regulated by specific sorting of newly synthesized products into secretory lysosomes and by stimulus-dependent vesicular/membrane biogenesis.9 Constitutive sorting of soluble cargo proteins from the Golgi to the endosomal/lysosomal system is mediated by mannose 6-phosphate receptors CD-MPR and CI-MPR, 2 major sorting receptors in numerous cell types.10,11 Cell-type–specific mechanisms for the selective delivery of cargo proteins to lysosomes, however, have hitherto remained elusive. Recently, we have presented evidence for the hypothesis that Th2-polarized macrophages use specific, stimulus-dependent mechanisms for protein delivery from the Golgi compartments to the endosomal/lysosomal system, where the selection of the cargo is mediated by stabilin-1.12
Stabilin-1 is a type 1 transmembrane receptor previously identified by us that shows a unique cell and tissue distribution.13,14 Stabilin-1 is a marker for alternative macrophage activation2 ; it is expressed by specialized tissue macrophages in placenta, skin, gut, and pancreas; in cardiac and skeletal muscle; and by sinusoidal endothelial cells in liver, spleen, bone marrow, and lymph nodes.2,15 In vitro, the expression of stabilin-1 can be induced in monocyte-derived macrophages by stimulation with interleukin-4 (IL-4) and dexamethasone, but not interferon γ (IFNγ). Stabilin-1 functions as an endocytic receptor in endothelial cells and macrophages; its ligand repertoire, however, differs from that of its closest homolog, stabilin-2.12,16 While stabilin-2 has been shown to be a specialized scavenger receptor of sinusoidal endothelial cells mediating uptake of hyaluronic acid, AGE, and procollagen peptides, the only well-established ligand for stabilin-1 to date is acLDL.17,18
In addition to endocytosis/recycling, stabilin-1 is involved in trafficking between early/sorting endosomes and the trans-Golgi network12 in human macrophages. Shuttling of stabilin-1 between the biosynthetic and endosomal/lysosomal compartments is mediated by GGAs, which bind to the DDSLL motif in the cytoplasmic tail of stabilin-1. GGAs are highly specialized adaptors responsible for TGN/endosomal shuttling of CI-MPR and sortilin that combine endocytic and sorting functions.10,19,20 This dual function of CI-MPR includes receptor-mediated endocytosis of insulin-like growth factor-II, and sorting of newly synthesized lysosomal hydrolases from the Golgi compartment to late endosomes followed by delivery to lysosomes.10
Since our data indicated the possibility of such a dual function for stabilin-1 (ie, scavenging of extracellular ligands and sorting of newly synthesized cargo from TGN to endosomal/lysosomal system), we searched for stabilin-1 interacting proteins. Yeast 2-hybrid screening of a human placental cDNA library resulted in the identification of the novel chitinase-like protein SI-CLP as a stabilin-1 interacting partner, and the interaction was confirmed independently by in vitro pull-down assays. SI-CLP gene expression was up-regulated by IL-4 and dexamethasone on the mRNA and protein levels in primary human macrophages in parallel with the expression of stabilin-1. We generated a rat monoclonal anti–SI-CLP antibody with which we demonstrated that the major sites of SI-CLP intracellular localization are CD63+ secretory lysosomes, and with which we detected secretion of SI-CLP in macrophages. Colocalization of stabilin-1 with SI-CLP in the TGN, stabilin-1–mediated relocalization of SI-CLP in H1299 cells, and impaired lysosomal sorting of SI-CLP in stabilin-1–deficient MϕIL-4/Dex together indicate that stabilin-1 may be involved in the delivery of newly synthesized SI-CLP from the biosynthetic compartment to the endosomal/lysosomal system. Using the anti–SI-CLP antibody 1C11, we demonstrated that IL-4 and dexamethasone in combination increase SI-CLP protein levels in macrophages, the extent of which varied among donors. In vivo, the 1C11 mAb recognized SI-CLP in the cellular fraction of bronchoalveolar lavage specimens obtained from patients with chronic inflammatory disorders of the respiratory tract and in peripheral-blood leukocytes (PBLs) from these patients as well as healthy donors.
Patients, materials, and methods
Isolation of peripheral-blood leukocytes and cells from bronchoalveolar lavage
Bronchoalveolar lavage (BAL) samples were obtained from patients with various types of respiratory tract disorders from the Department of Pulmonology, Theresien Hospital, Mannheim, Germany. The study was approved by the Medical Ethics Committee II of the Faculty of Clinical Medicine Mannheim, University of Heidelberg. Informed consent was provided according to the Declaration of Helsinki. Cells were isolated from the lavage suspension by centrifugation. Blood samples were obtained from the same patients in parallel and from healthy volunteers. PBLs were isolated from heparin blood using Ficoll gradient centrifugation. Both BAL cells and PBLs were used for Western blot sample preparation by direct lysis of cell pellets in Laemmli loading buffer under reducing conditions. Alternatively, cell pellets were aliquoted and stored at –80°C.
Primary macrophages and cell lines
Human monocytes/macrophages were isolated and cultivated as described.12 The cells were purified from individual buffy coats by density gradient centrifugations. Monocyte-enriched fractions were subjected to positive CD14+ Magnetic Cell Sorting using CD14 Magnetic beads (Miltenyi Biotech, Bergisch Gladbach, Germany), resulting in 90% to 98% monocyte purity as confirmed by flow cytometry.
Macrophages were cultivated in X-VIVO 10 Serum-free Medium (Cambrex, Verviers, Belgium) at a concentration of 1 × 106 cells/mL with the following stimulants: IFNγ at 1000 U/mL; IL-4 and IL-10 at 10 ng/mL (TEBU Bio, Frankfurt am Main, Germany); and dexamethasone at 1 × 10–7 M (Sigma, Munich, Germany).
THP-1, U-937, and Mono-Mac-6 cells were propagated in X-VIVO 10 Serum-free Medium. A-549, HeLa, HEK-293, H1299, and MCF-7 cell lines were propagated in DMEM (Invitrogen, Karlsruhe, Germany) supplemented with 10% FCS (Biochrom, Berlin, Germany) and penicillin/streptomycin. Jurkat and Raji cells were propagated in RPMI (Invitrogen) supplemented with 10% FCS. H1299-SI-CLP-FLAG cells stably transfected with IRESneo-SI-CLP-FLAG were propagated in the presence of G418.
Transfection with siRNA
After 4 days in culture, macrophages were transfected with 1.5 μg annealed siRNA per 5 × 106 cells using a Nucleofector Device (program Y-001) and a Human Monocyte Nucleofector kit (Amaxa, Koeln, Germany). Predesigned stabilin-1 siRNA (ID136774) and Silencer Negative Control no. 1 were from Ambion (Cambridge, United Kingdom).
Yeast 2-hybrid screening
The yeast 2-hybrid screen was performed using the Matchmaker Gal4 2-Hybrid system (BD Clontech, Heidelberg, Germany). For bait generation, a fragment of stabilin-1 cDNA corresponding to amino acids 2302 to 2570 was amplified by polymerase chain reaction (PCR) and cloned in the pDNR2 vector followed by subcloning in pLP-GBKT7 using the BD Creator cloning kit (BD Clontech). The bait protein was characterized according to the manufacturer's protocols; it was expressed on a high level, did not activate transcription in yeast, and did not affect the mating efficiency. The pretransformed human placenta Matchmaker cDNA library in the pGADT7-Rec vector, the Yeastmaker Yeast Transformation kit, and plasmid isolation kits were purchased from BD Clontech. Selection conditions of the highest stringency were used.
SI-CLP expression constructs and transfections
The IMAGEp958P22178Q2 clone containing full-length cDNA for SI-CLP was obtained from RZPD (Berlin, Germany) and used to generate SI-CLP expression constructs. The expression construct pcDNA3-SI-CLP was created by inserting full-length SI-CLP cDNA between the EcoRI and XhoI sites of pcDNA3HisC (Invitrogen). The expression construct pLP-IRESneo-SI-CLP-FLAG was made with a BD Creator DNA cloning kit. Full-length SI-CLP cDNA was cloned between the EcoRI and XhoI sites of pDNR2; the FLAG epitope coding sequence was introduced by adaptor cloning into the XhoI site; and the resulting SI-CLP-FLAG fusion cDNA was cloned into pLP-IRESneo by Cre recombination. For bacterial expression, pGEX-SI-CLP-A was constructed by cloning the SI-CLP fragment corresponding to amino acids 24 to 73 into pGEX4T1 (Invitrogen). pET-His-SI-CLP was constructed by cloning full-length SI-CLP cDNA into PET-28a(+) (Novagen, Madison, WI). H1299 cells were transiently transfected with Lipofectamine (Invitrogen). Cells were used for immunofluorescence (IF) 36 hours after transfection or for lysate preparation 48 hours after transfection. The H1299-SI-CLP cell line was generated by stable transfection of H1299 cells with the pLP-IRESneo-SI-CLP-FLAG construct.
Anti–SI-CLP antibody generation
A fragment of SI-CLP corresponding to amino acids 24 to 73 was expressed as a GST fusion protein in E coli strain BL21-CodonPlus-RIL (Stratagene, La Jolla, CA), purified under native conditions as described,21 and used to generate rat mAbs. Specificity of the mAbs was tested for Western blot using the following immunogens: purified His-tagged full-length SI-CLP from E coli, and the cell lysates of H1299 transiently and stably transfected with pLP-IRESneo-SI-CLP-FLAG. Reactivity of the mAbs for immunofluorescence was examined using H1299 cells transfected with pLP-IRESneo-SI-CLP-FLAG and nontransfected H1299 cells as a negative control (data not shown). The mAb 1C11 (isotype IgG2a) was selected for further study.
In vitro protein-protein interactions
GST pull-down assays were performed as described.12 Fragments of stabilin-1 corresponding to amino acids 2302 to 2570 (P9) and 2327 to 2463 (F7) were cloned into the EcoRI/XhoI sites of the pGEX4T1 vector (Invitrogen). GST-fused proteins were expressed in E coli strain BL21-CodonPlus-RIL (Stratagene) and purified under nondenaturing conditions. PcDNA3-SI-CLP was used as a template to generate 35S-methionine–labeled proteins by in vitro translation according to the standard protocol (Promega, Mannheim, Germany).
Immunofluorescence and confocal microscopy
Cell fixation and staining procedures were performed as described.12 The following primary antibodies were used: rabbit polyclonal anti–stabilin-1 F414 ; rat monoclonal anti–SI-CLP, rat IgG2a isotype control (eBiosciences, San Diego, CA), rat IgG (Dianova, Hamburg, Germany), anti-Lamp1, and anti-Lamp3 (to be consistent; Santa Cruz Biotechnology, Santa Cruz, CA); and anti-p62lck (BD Clontech). Secondary antibodies were Cy2-conjugated donkey anti–mouse IgG (Dianova), Cy3-conjugated goat anti–rat IgG (Dianova), and Alexa488-conjugated goat anti–rabbit IgG (Mobitec, Göttingen, Germany). Samples were mounted using DakoCytomation Fluorescent Mounting Medium (DakoCytomation, Hamburg, Germany). Confocal microscopy was performed using a Leica laser scanning spectral confocal microscope, model DM IRE2, equipped with an HCX PL Apo 63 ×/1.32 numeric aperture oil objective (Leica Microsystems, Wetzlar, Germany). Excitation was with an argon laser emitting at 488 nm, a krypton laser emitting at 568 nm, and a helium/neon laser emitting at 633 nm. Images were acquired using a TCS SP2 scanner and Leica Confocal software, version 2.5 (both from Leica Microsystems). All 2-color images were acquired using a sequential scan mode. For panel assembly, Adobe Photoshop version 6.0 (Adobe Systems, San Jose, CA) and Corel Draw version 10 (Corel, Ottawa, ON, Canada) were used.
Real-time RT-PCR analysis
Real-time reverse-transcriptase (RT)–PCR analysis of YKL-39 and YKL-40 expression was performed using the SYBR Green PCR Master Mix. The primers for YKL-39 were forward 5′-AAGATGACCTTGCTGCCT and reverse 5′-TGATCTAAGAGGAAGTCAGG; for YKL-40, forward 5′-CCTATGCAGAGGTCCACAAC and reverse 5′-GAGTCTTACATTGCGATGCC. Real-time PCR analysis of SI-CLP expression was performed using SI-CLP TaqMan Assay Hs00388156_m1 together with TaqMan PCR Master Mix. The TaqMan probe for human GAPDH was used as an internal control. The experiments were performed on the ABI PRISM 7000 sequence detection system. The reagents and equipment for real-time RT-PCR were supplied by Applied Biosystems (Darmstadt, Germany).
Western blot analysis
Samples for Western blotting were prepared by direct lysis of the cells in Laemmli loading buffer supplemented with β-mercaptoethanol. Conditioned media were concentrated 10 times in Vivaspin concentrators (Vivascience/Sartorius, Göttingen, Germany) and mixed with equal volumes of Laemmli buffer. Protein loading was controlled by parallel gel staining with GelCode Blue Stain Reagent (Pierce, Rockford, IL). Blots were incubated either with 1C11 or with control antibody (purified rat IgG [Dianova] or rat isotype control IgG2a [eBiosciences]) at 2.4 μg/mL, followed by incubation with HRP-conjugated goat anti–rat Fab2 (Amersham Biosciences, Freiburg, Germany). Pierce Super Signal Pico system was used for signal detection. In several cases, lysate loading was additionally controlled by GAPDH detection (data not shown).
Results
Identification of the novel human chitinase-like protein SI-CLP as an interacting partner of stabilin-1
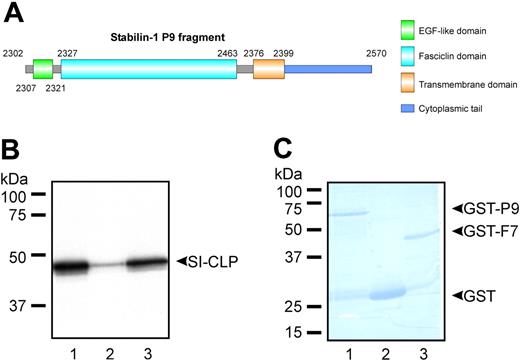
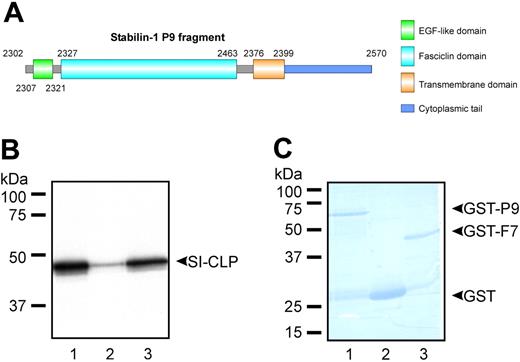
Stabilin-1 is a type-1 transmembrane protein composed of a short cytoplasmic tail that interacts with GGA adaptors and a large extracellular part with a highly complex structure comprising 7 fasciclin and multiple EGF-like domains potentially involved in various protein-protein interactions.12,14 We applied yeast 2-hybrid screening for identification of proteins interacting with stabilin-1. The stabilin-1 bait fragment P9 did not activate transcription and was expressed at a high level in yeast. The P9 fragment contains one EGF-like domain, the final fasciclin domain (F7), the transmembrane region, and the cytoplasmic tail (Figure 1A). Screening of a human placental cDNA library under conditions of highest stringency resulted in identification of the novel protein SI-CLP as an interacting partner of stabilin-1. The sequence of the identified fragment demonstrated 100% similarity to the hypothetical protein GL008 (GenBank accession no. AF212229)/IMAGE clone 3504261 (GenBank accession no. BC000001). The coding sequence of SI-CLP expressed by primary human macrophages was 100% identical to that of BC000001 and encoded a protein with a predicted molecular weight of 44.9 kDa. The sequence was deposited in GenBank under the accession number BN000479. Interaction of the full-length SI-CLP protein with the bacterially expressed P9 fragment was confirmed independently by GST pull-down assays and was found to be mediated by the stabilin-1 fasciclin domain 7 (Figure 1B-C).
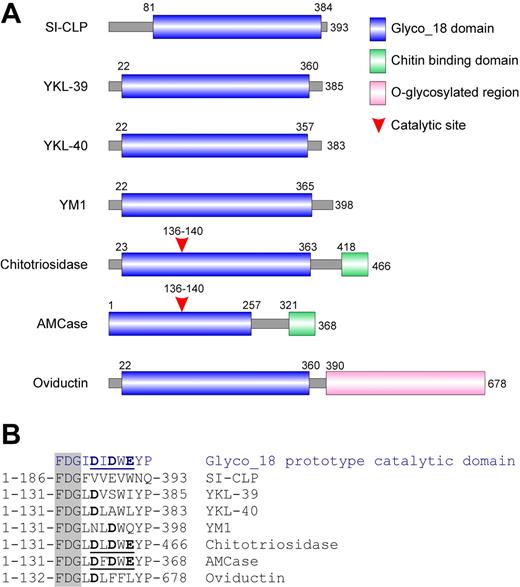
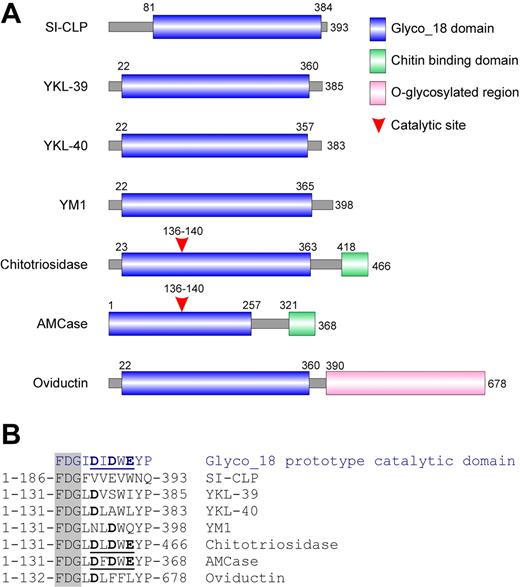
Analysis of the domain organization of SI-CLP revealed that its major part contains a conserved Glyco_18 domain. This domain is highly specific for chitinases belonging to the family 18 glycosyl hydrolases found in bacteria, plants, fungi, insects, viruses, and protozoan parasites22 as well as in mammals. Chitinases are chitinolytic enzymes with substrate-assisted activity. Glyco_18 domain–containing proteins are also present in higher eukaryotes. Until now, 5 human Glyco_18 domain–containing proteins have been identified. These are chitotriosidase, AMCase, YKL-39, YKL-40, and oviductin/MUC9. Additionally, the closely related proteins YM1 and YM2 have been reported in rodents. A comparative analysis of the domain organization of SI-CLP with other mammalian Glyco_18 domain–containing proteins is presented in Figure 2. SI-CLP (as well as YKL-39, YKL-40, and YM1/YM2) contains a Glyco_18 domain but, in contrast to chitotriosidase and AMCase, does not contain a chitin-binding domain (Figure 2A). Oviductin/MUC9 contains a Glyco_18 domain and a long fragment with numerous sites for O-glycosylation, characteristic of mucins. The catalytic activity of Glyco_18 domain proteins is defined by the presence of a catalytic glutamic acid in the context of a DXDXE site located directly (with one amino acid as a spacer) after the characteristic amino acid triplet FDG. While chitotriosidase and AMCase are true chitinases with a classic catalytic site, enzymatic activity, and the ability to bind chitin through their C-terminal chitin-binding domain, YKL-39, YKL-40, YM1/YM2, and oviductin lack critical amino acids within the catalytic site (Figure 2B) and do not exhibit enzymatic activity.23 Analysis of the Glyco_18 domain of SI-CLP revealed that the FDG sequence is present at positions 186 to 188. However, there is no characteristic DXDXE domain after the FDG sequence (Figure 2B), indicating the absence of catalytic activity of the SI-CLP Glyco_18 domain.
Identification of SI-CLP as stabilin-1 interacting protein. (A) Schematic representation of stabilin-1 fragment used as a bait in yeast 2-hybrid screening. (B) GST pull-down assay using bacterially expressed GST-P9 (lane 1), GST (lane 2), and GST-F7 (lane 3). 35S-labeled full-length SI-CLP specifically interacts with P9 fragment and fasciclin 7 domain of stabilin-1. (C) Control of GST-fused protein amounts used in pull-down reaction.
Identification of SI-CLP as stabilin-1 interacting protein. (A) Schematic representation of stabilin-1 fragment used as a bait in yeast 2-hybrid screening. (B) GST pull-down assay using bacterially expressed GST-P9 (lane 1), GST (lane 2), and GST-F7 (lane 3). 35S-labeled full-length SI-CLP specifically interacts with P9 fragment and fasciclin 7 domain of stabilin-1. (C) Control of GST-fused protein amounts used in pull-down reaction.
SI-CLP is a novel member of Glyco_18 domain–containing human chitinases and chitinase-like proteins. (A) Schematic representation of domain organization of mammalian Glyco_18 domain–containing protein. (B) Critical amino acid in catalytic sites. The characteristic FDG sequence preceding catalytic motif is shown in shaded column. Catalytic amino acids are shown in bold. Complete active catalytic motifs are underlined.
SI-CLP is a novel member of Glyco_18 domain–containing human chitinases and chitinase-like proteins. (A) Schematic representation of domain organization of mammalian Glyco_18 domain–containing protein. (B) Critical amino acid in catalytic sites. The characteristic FDG sequence preceding catalytic motif is shown in shaded column. Catalytic amino acids are shown in bold. Complete active catalytic motifs are underlined.
According to its domain organization and the absence of catalytic amino acids, SI-CLP can be grouped with YKL-39, YKL-40, and YM1/YM2. However, SI-CLP is the least homologous member of the human Glyco_18 domain–containing protein family. Comparison of Glyco_18 domains using the BLAST algorithm revealed that the Glyco_18 domain of SI-CLP has 20% identity with the Glyco_18 domain of YKL-39, while the identity of the Glyco_18 domains for the other proteins ranges from 43% to 68%. Characteristically, the Glyco_18 domains of most of the previously described human proteins immediately follow the signal peptide sequence (positions 22-23 in the full-length protein sequence) (Figure 2A). SI-CLP differs from other Glyco_18 domain–containing proteins by the presence of a unique region between the putative signal peptide and the beginning of the Glyco_18 domain (Figure 2A). We used this unique protein fragment to generate rat mAbs specific to SI-CLP, one of which we selected for its ability to recognize by Western blot the bacterially expressed recombinant proteins GST-SI-CLP-A (aa 24-73) and His-SI-CLP full-length protein, as well as full-length FLAG-tagged SI-CLP expressed in mammalian cells (data not shown and Figure 3). The same clone, 1C11, recognized SI-CLP-FLAG in both transiently and stably transfected H1299 cells by immunofluorescence (data not shown). This clone was used for further analysis of SI-CLP expression and localization in human macrophages and other cell types.
SI-CLP protein expression in cells of monocytic, B, T, and epithelial origin
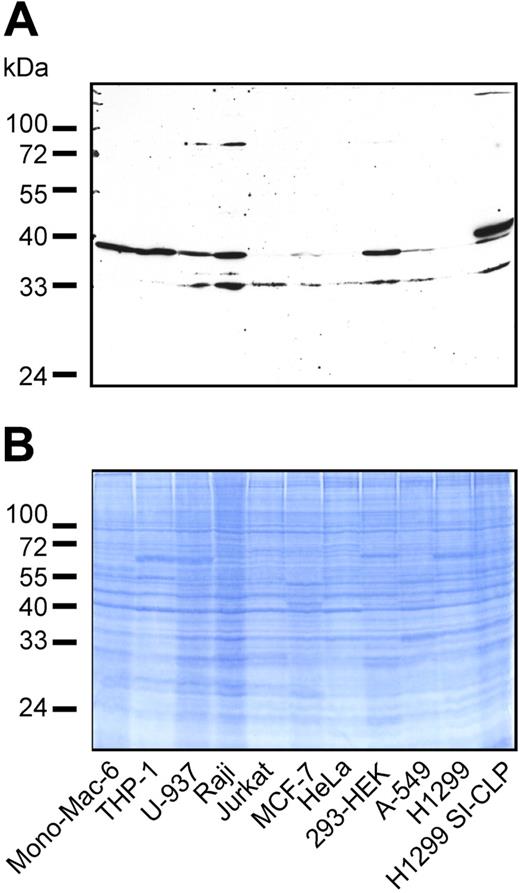
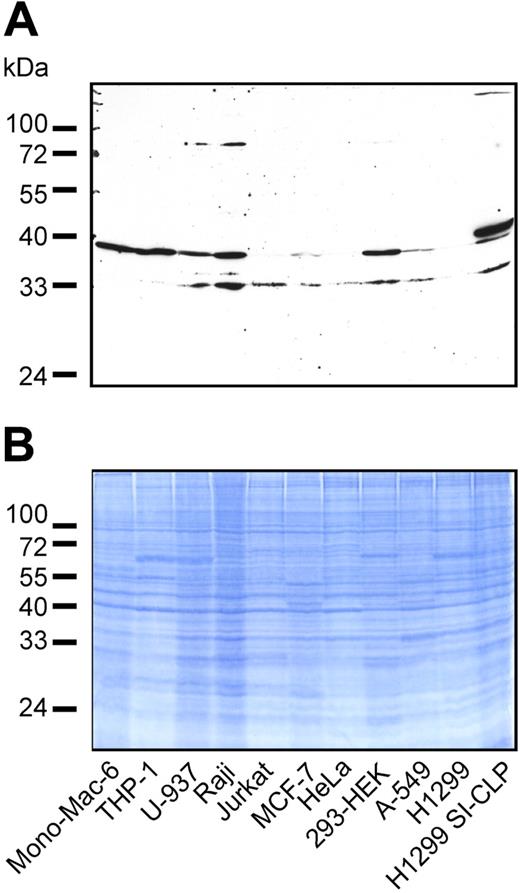
Several mammalian Glyco_18 domain–containing proteins were found not only in macrophages but also in B and T cells and in cells of epithelial origin. To analyze whether SI-CLP expression is restricted to cells of monocytic origin, we examined several cell lines by Western blotting with 1C11. SI-CLP was represented by 2 bands of about 33 and 39 kDa. The pattern of SI-CLP isoforms was cell-line specific (Figure 3). All 3 cell lines of monocytic origin (ie, Mono-Mac-6, THP-1, and U-937) expressed high amounts of SI-CLP. Comparable levels of SI-CLP expression were found in the B-cell–derived cell line Raji and in the embryonal kidney fibroblastoid cell line HEK-293. Low levels of SI-CLP expression were detected in the T-cell–derived cell line Jurkat and tumor cell lines of epithelial origin, including MCF-7, A-549, and HeLa. H1299 cells yielded a barely detectable signal and can be assumed to be negative (Figure 3). The molecular weight of the recombinant SI-CLP-FLAG expressed in transfected H1299 cells was slightly larger than the 39-kDa band of the endogenous protein. These data indicate that SI-CLP is expressed in cells of monocytic, T, B, and epithelial origin. The difference in molecular weight of the endogenous and recombinant proteins suggests cell type–specific processing of SI-CLP similar to that of human chitotriosidase.24
SI-CLP expression is up-regulated by IL-4 and dexamethasone in macrophages
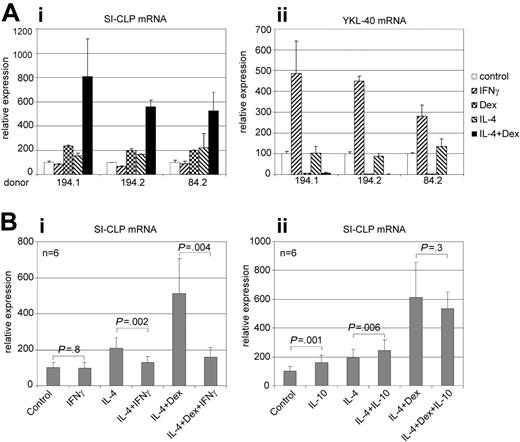
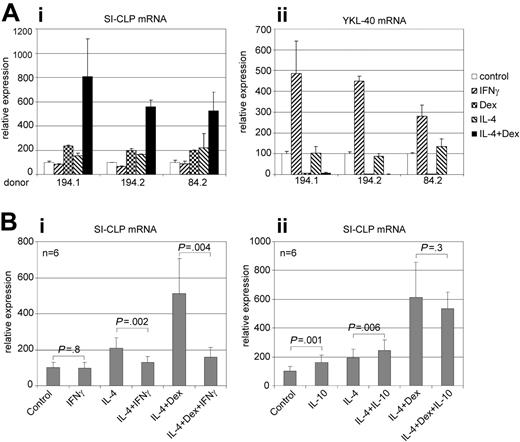
Several Glyco_18 domain–containing proteins (ie, chitotriosidase, AMCase, YKL-40, and YM1) are produced by macrophages25-28 ; expression of AMCase and YM1 in macrophages was found to be associated with Th2 cytokine context. Here, we analyzed the regulation of SI-CLP expression in primary human macrophages. Monocyte-derived macrophages were stimulated by IFNγ, IL-4, dexamethasone, and a combination of IL-4 and dexamethasone for 6 days; the expression of SI-CLP was analyzed using real-time RT-PCR. SI-CLP from all donors for this assay was induced by both IL-4 and dexamethasone. The highest level of SI-CLP mRNA expression was detected in macrophages stimulated by the combination of IL-4 and dexamethasone, indicating synergistic effects of these stimuli. However, the strength of this synergistic effect varied among donors. Stimulation with IFNγ had no effect on SI-CLP mRNA expression (Figure 4Ai). Next, we analyzed whether IFNγ or IL-10 is able to modulate the effect of IL-4 and dexamethasone. Analysis of macrophages derived from 6 independent donors revealed that IFNγ efficiently suppresses IL-4/dexamethasome (Dex)–induced SI-CLP expression. IL-10 has a weak but statistically significant stimulatory effect alone or in combination with IL-4, but has no statistically significant effect on IL-4/Dex stimulation (Figure 4B).
Expression of SI-CLP protein in human cell lines. (A) Western blot analysis of total cell lysates using rat mAb 1C11. Endogenous SI-CLP is represented by specific bands corresponding to MW of about 39 kDa and 33 kDa. In stable transfected H1299-SI-CLP cells, the strongest SI-CLP band has a MW of about 40 kDa. None of these bands is recognized by isotype control rat IgG2a and rat IgGs (data not shown). (B) Loading control.
Expression of SI-CLP protein in human cell lines. (A) Western blot analysis of total cell lysates using rat mAb 1C11. Endogenous SI-CLP is represented by specific bands corresponding to MW of about 39 kDa and 33 kDa. In stable transfected H1299-SI-CLP cells, the strongest SI-CLP band has a MW of about 40 kDa. None of these bands is recognized by isotype control rat IgG2a and rat IgGs (data not shown). (B) Loading control.
Real-time RT-PCR analysis of SI-CLP and YKL-40 expression in human macrophages. Peripheral blood–derived monocytes nonstimulated (control) or stimulated with cytokines as indicated were propagated in culture for 6 days. (A) Three representative donors with differential responsivities are presented. (Ai) IL-4, dexamethasone, and combination of both induce SI-CLP mRNA up-regulation in macrophage cultures; the lowest SI-CLP expression is detected in case of IFNγ stimulation. (Aii) IFNγ induces YKL-40 mRNA expression, whereas dexamethasone has strong inhibitory effect. (B) Effect of IFNγ (i) and IL-10 (ii) on the IL-4/Dex-induced expression of SI-CLP. Peripheral blood–derived monocytes of 6 independent donors were used for each analysis. The data were analyzed using paired 2-tailed t test. The difference was considered to be statistically significant in case of P < .05. IFNγ suppresses the effect of IL-4/Dex. IL-10 has no statistically significant effect on IL-4/Dex stimulation. The expression levels of analyzed genes were normalized to GAPDH mRNA expression. Error bars represent SD.
Real-time RT-PCR analysis of SI-CLP and YKL-40 expression in human macrophages. Peripheral blood–derived monocytes nonstimulated (control) or stimulated with cytokines as indicated were propagated in culture for 6 days. (A) Three representative donors with differential responsivities are presented. (Ai) IL-4, dexamethasone, and combination of both induce SI-CLP mRNA up-regulation in macrophage cultures; the lowest SI-CLP expression is detected in case of IFNγ stimulation. (Aii) IFNγ induces YKL-40 mRNA expression, whereas dexamethasone has strong inhibitory effect. (B) Effect of IFNγ (i) and IL-10 (ii) on the IL-4/Dex-induced expression of SI-CLP. Peripheral blood–derived monocytes of 6 independent donors were used for each analysis. The data were analyzed using paired 2-tailed t test. The difference was considered to be statistically significant in case of P < .05. IFNγ suppresses the effect of IL-4/Dex. IL-10 has no statistically significant effect on IL-4/Dex stimulation. The expression levels of analyzed genes were normalized to GAPDH mRNA expression. Error bars represent SD.
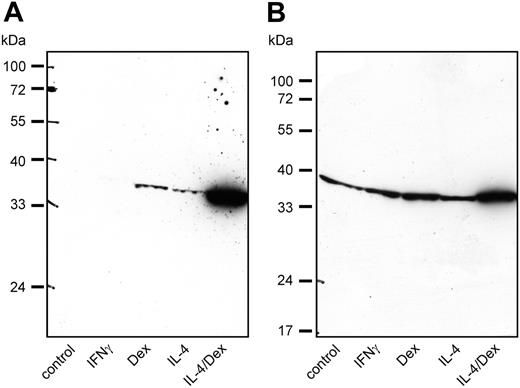
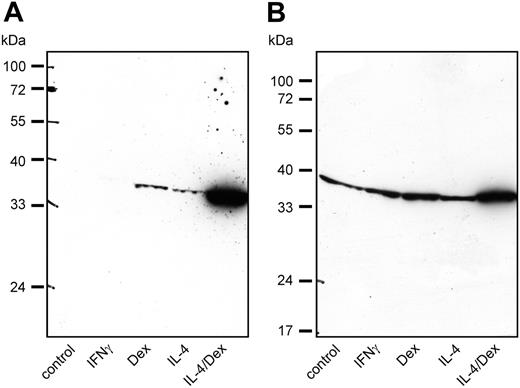
To demonstrate the expression of SI-CLP protein in macrophages, we used rat mAb 1C11. Western blot analysis of macrophage total cell lysates confirmed that SI-CLP expression is inducible by IL-4 or dexamethasone. While dexamethasone stimulation led to slightly higher SI-CLP levels than IL-4, the strongest SI-CLP induction was achieved by stimulation with a combination of IL-4 and dexamethasone. The extent of SI-CLP up-regulation differed substantially among individual donors (Figure 5). The effects of IFNγ and IL-10 on SI-CLP expression shown in Figure 4B were confirmed on the protein level (data not shown). Comparison of the SI-CLP protein level in macrophages with that of freshly isolated monocytes revealed that the differentiation of monocytes into macrophages in vitro resulted in an increase of SI-CLP expression (data not shown). Notably, only the band of about 39 kDa was detected in all populations of macrophages.
Among human Glyco_18 domain–containing proteins, YKL-39 and YKL-40 proteins possess the domain organization with the highest similarity to that of SI-CLP. YKL-40, but not YKL-39, was shown to be produced by macrophages.27 Therefore, we examined whether Th1 and Th2 cytokines or dexamethasone influence the expression of YKL-39 or YKL-40 in human macrophages. Using RT-PCR analysis, we demonstrated that YKL-39 and YKL-40 transcripts are present in all macrophage populations tested (data not shown). The quantitative analysis of YKL-39 and YKL-40 expression was also performed by real-time RT-PCR. We demonstrated that YKL-40 was dramatically up-regulated by IFNγ. Dexamethasone had a strong suppressive effect on YKL-40 mRNA expression; stimulation by IL-4 was not able to prevent this effect (Figure 4Aii). YKL-39 was expressed on a very low level in all macrophage subpopulations tested; and no specific effects of IFNγ, IL-4, or dexamethasone were detected (data not shown). We concluded that SI-CLP is the only Glyco_18 domain–containing human protein whose expression is stimulated by dexamethasone or IL-4 alone or in combination and that the level of its up-regulation in alternatively activated macrophages varies among donors.
SI-CLP is a lysosomal secreted protein
Mammalian Glyco_18 domain–containing proteins include catalytically active chitinases and chitinase-like proteins with cytokine activity. Macrophages are professional secretory cells and they use the lysosomal-derived vesicular pathway to secrete several classes of products such as lysosomal hydrolases. It was reported that chitotriosidase is sorted to lysosomes in human macrophages.24 Therefore, we examined whether SI-CLP is also sorted into secretory lysosomes in macrophages. Macrophages were stimulated with IL-4 and dexamethasone to achieve a high level of SI-CLP expression and analyzed by indirect immunofluorescence and confocal microscopy. We found that a substantial proportion of intracellular SI-CLP is localized in Lamp1-positive classic lysosomes (Figure 6A). This high level of SI-CLP–Lamp1 colocalization was detected in all isolations of primary macrophages and in Mono-Mac-6 cells (data not shown). To identify whether SI-CLP is targeted for secretion, we examined SI-CLP colocalization with CD63, known to be specifically associated with a subpopulation of secretory lysosomes.7,29 We found that SI-CLP is indeed present in CD63+ lysosomes (Figure 6B). Newly synthesized proteins targeted to the endosomal/lysosomal system use receptor-mediated trafficking and can be delivered to late endosomes for further trafficking to lysosomes, as described for MPR-mediated sorting of lysosomal hydrolases.10 Since p62lck has been shown to function in lysosome-targeted late endosomes,30 we used it as a marker to determine whether SI-CLP is delivered primarily to late endosomes. Double immunofluorescence revealed that a part of the SI-CLP colocalizes in late endosomes with p62lck in IL-4/Dex–stimulated macrophages as well as in Mono-Mac-6 cells (Figure 6C and data not shown).
SI-CLP protein level is up-regulated in human macrophages by IL-4 and dexamethasone. Western blot analysis with 1C11 rat mAb shows individual differences in response to various stimulations. (A) Macrophages derived from donor 140.3 show strong up-regulation of SI-CLP under stimulation with IL-4 and dexamethasone. (B) Macrophages derived from donor 147.3 show intermediate-level SI-CLP up-regulation under stimulation with IL-4 and dexamethasone.
SI-CLP protein level is up-regulated in human macrophages by IL-4 and dexamethasone. Western blot analysis with 1C11 rat mAb shows individual differences in response to various stimulations. (A) Macrophages derived from donor 140.3 show strong up-regulation of SI-CLP under stimulation with IL-4 and dexamethasone. (B) Macrophages derived from donor 147.3 show intermediate-level SI-CLP up-regulation under stimulation with IL-4 and dexamethasone.
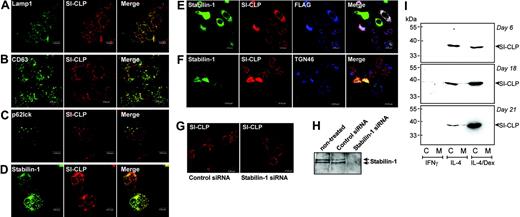
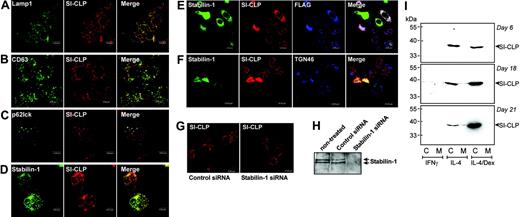
Intracellular distribution of SI-CLP in human macrophages. Peripheral blood–derived monocytes were stimulated with IL-4 and dexamethasone for 6 days and used for immunofluorescent/confocal microscopy examination. SI-CLP was detected with rat mAb 1C11/antirat Cy3-conjugated secondary ab (red). Other proteins are visualized in green. Merge of green and red is shown in yellow; red and blue, in pink; and green, red, and blue, in white. (A) SI-CLP strongly colocalizes with Lamp1. (B) SI-CLP strongly colocalizes with CD63, marker for secretory lysosomes. (C) SI-CLP is found in p62lck-positive late endosomes. (D) SI-CLP partially colocalizes with stabilin-1 in TGN but is absent from stabilin-1–positive early endosomes. (E-F) Recombinant FLAG-tagged SI-CLP is missorted in globular structures localized in nuclear area (controlled by Dapi) in stably transfected H1299 cells. 1C11-positive structures did not colocalize with lysosomal markers (not shown). Transient overexpression of stabilin-1 resulted in relocalization of SI-CLP into the cytoplasm. Stabilin-1 and SI-CLP partially colocalize with TGN46. (G) MϕIL-4/Dex were transfected with control siRNA and stabilin-1 siRNA on day 4 of culture. Intracellular distribution of SI-CLP was analyzed on day 6 (2 days after transfection). The decreased sorting into lysosomes and abnormal concentration in nuclear rim structures were detected in part of cells transfected with stabilin-1 siRNA. (H) Stabilin-1 protein expression in MϕIL-4/Dex is efficiently suppressed by stabilin-1 siRNA. Western blot analysis with anti–stabilin-1 F4 ab. (I) Western blot analysis of SI-CLP secretion in long-term macrophage cultures. SI-CLP is detected in conditioned medium of IL-4–stimulated macrophages after 18 and 21 days of stimulation. Costimulation with dexamethasone results in intracellular accumulation of SI-CLP but blocks its secretion. The absence of cell damage was controlled by quantification of lactate dehydrogenase (LDH) activity in conditioned medium using a cytotoxicity detection kit (Roche, Mannheim, Germany).
Intracellular distribution of SI-CLP in human macrophages. Peripheral blood–derived monocytes were stimulated with IL-4 and dexamethasone for 6 days and used for immunofluorescent/confocal microscopy examination. SI-CLP was detected with rat mAb 1C11/antirat Cy3-conjugated secondary ab (red). Other proteins are visualized in green. Merge of green and red is shown in yellow; red and blue, in pink; and green, red, and blue, in white. (A) SI-CLP strongly colocalizes with Lamp1. (B) SI-CLP strongly colocalizes with CD63, marker for secretory lysosomes. (C) SI-CLP is found in p62lck-positive late endosomes. (D) SI-CLP partially colocalizes with stabilin-1 in TGN but is absent from stabilin-1–positive early endosomes. (E-F) Recombinant FLAG-tagged SI-CLP is missorted in globular structures localized in nuclear area (controlled by Dapi) in stably transfected H1299 cells. 1C11-positive structures did not colocalize with lysosomal markers (not shown). Transient overexpression of stabilin-1 resulted in relocalization of SI-CLP into the cytoplasm. Stabilin-1 and SI-CLP partially colocalize with TGN46. (G) MϕIL-4/Dex were transfected with control siRNA and stabilin-1 siRNA on day 4 of culture. Intracellular distribution of SI-CLP was analyzed on day 6 (2 days after transfection). The decreased sorting into lysosomes and abnormal concentration in nuclear rim structures were detected in part of cells transfected with stabilin-1 siRNA. (H) Stabilin-1 protein expression in MϕIL-4/Dex is efficiently suppressed by stabilin-1 siRNA. Western blot analysis with anti–stabilin-1 F4 ab. (I) Western blot analysis of SI-CLP secretion in long-term macrophage cultures. SI-CLP is detected in conditioned medium of IL-4–stimulated macrophages after 18 and 21 days of stimulation. Costimulation with dexamethasone results in intracellular accumulation of SI-CLP but blocks its secretion. The absence of cell damage was controlled by quantification of lactate dehydrogenase (LDH) activity in conditioned medium using a cytotoxicity detection kit (Roche, Mannheim, Germany).
We demonstrated recently that stabilin-1 is involved in 2 major intracellular trafficking pathways in human macrophages: (1) endocytosis/recycling and (2) shuttling between endosomal compartments and TGN.12 To identify the putative site of stabilin-1 interaction with SI-CLP, we analyzed whether SI-CLP is present in stabilin-1–positive structures. Since stabilin-1 does not enter Lamp1-positive lysosomes and only transiently enters p62lck-positive late endosomes,12 the localization of SI-CLP in the major stabilin-1–positive intracellular compartments (ie, early endosomes and TGN) was studied. SI-CLP was not found in EEA1-positive stabilin-1–containing endosomes (data not shown). However SI-CLP was found to partially colocalize with stabilin-1 in the trans-Golgi network (Figure 6D). The extent of colocalization differed between individual macrophage preparations and between single macrophages in each culture, indicating that this is a transient and stimulation-dependent event. To test whether stabilin-1 is directly involved in SI-CLP sorting, we generated the H1299-SI-CLP-FLAG cell line, which lacks endogenous SI-CLP (Figure 3) and expresses recombinant FLAG-tagged SI-CLP (Figures 3 and 6E). SI-CLP-FLAG was missorted into the globular nuclear structures or localized at the nuclear rim in H1299 cells, in contrast to its lysosomal localization in all other tested cells. Transient overexpression of stabilin-1 resulted in complete relocalization of SI-CLP-F into stabilin-1–positive cytoplasmic structures, partially colocalized with TGN (Figure 6E-F). Knock-down of stabilin-1 by transfection of primary MϕIL4/Dex with stabilin-1 siRNA resulted in decreased sorting of SI-CLP to lysosomes (Figure 6G-H). We concluded that SI-CLP is delivered from the biosynthetic late Golgi compartments to lysosome-targeted late endosomes; thereafter, SI-CLP is delivered to secretory lysosomes. Our data also support the hypothesis that stabilin-1 is involved in routing of SI-CLP from the biosynthetic to the secretory pathway in IL-4/Dex-stimulated macrophages.
Long-term macrophage propagation revealed that SI-CLP is secreted into the culture medium (Figure 6I). Macrophages stimulated by IL-4 secreted SI-CLP after 18 days in culture. Addition of dexamethasone resulted in intracellular accumulation of SI-CLP. This is consistent with the ability of dexamethasone to block lysosomal secretion by reducing cytosolic pH and modulating PKC activity.8,31
SI-CLP is present in BAL cells and in PBLs
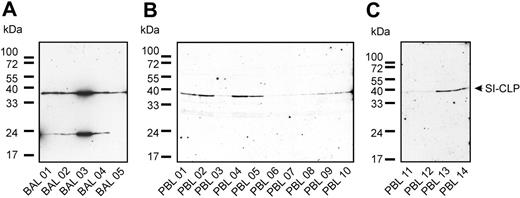
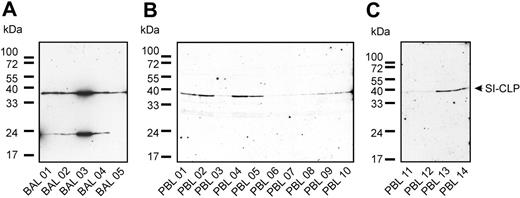
The recent finding that the Glyco_18 domain–containing protein AMCase is present in the BAL of ovalbumin-challenged mice (OVA asthma model)26 prompted us to examine the expression of SI-CLP in human BAL samples. The cellular fraction of BAL was analyzed by Western blot using 1C11 antibody (Figure 7A). SI-CLP protein was detected in all samples; however, expression levels varied. The highest SI-CLP expression was detected in patient no. 3 with sarcoidosis. The other patients with chronic bronchitis had similar levels of SI-CLP protein expression. In samples from 4 of 5 patients, we detected an additional band at approximately 23 kDa; this band was not recognized by the isotype control ab IgG2a or by rat IgG (Figure 7A and data not shown). The 1C11 mAb recognized a single 39-kDa band in peripheral-blood leukocytes of patients as well as of healthy donors (Figure 7B-C). Our data indicate that 1C11 can be used to analyze the expression level of SI-CLP in human BAL and PBLs.
1C11 monoclonal antibody detects SI-CLP in bronchoalveolar lavage samples and peripheral-blood leukocytes. Western blot analysis was performed using 1C11 mAb. (A) Different pattern of SI-CLP expression was observed in cells isolated out of bronchoalveolar lavage (BAL). BAL 01, 02, 04, and 05 are obtained from patients with chronic bronchitis; BAL 03 was obtained from patient with sarcoidosis, undergoing corticoid therapy. (B-C) PBLs were isolated from fresh blood samples by gradient centrifugation. PBL samples 01 to 05 correspond to BAL samples 01 to 05; PBL samples 06 to 14 are obtained from healthy donors. PBL samples 06 and 11 are obtained from the same healthy donor.
1C11 monoclonal antibody detects SI-CLP in bronchoalveolar lavage samples and peripheral-blood leukocytes. Western blot analysis was performed using 1C11 mAb. (A) Different pattern of SI-CLP expression was observed in cells isolated out of bronchoalveolar lavage (BAL). BAL 01, 02, 04, and 05 are obtained from patients with chronic bronchitis; BAL 03 was obtained from patient with sarcoidosis, undergoing corticoid therapy. (B-C) PBLs were isolated from fresh blood samples by gradient centrifugation. PBL samples 01 to 05 correspond to BAL samples 01 to 05; PBL samples 06 to 14 are obtained from healthy donors. PBL samples 06 and 11 are obtained from the same healthy donor.
Discussion
In the present study, we identified SI-CLP as a novel member of the mammalian family of Glyco_18 domain–containing proteins, comprising true enzymes as well as secreted chitinase-like proteins lacking enzymatic activity. While the hydrolytic activity of chitotriosidase and AMCase indicates their possible involvement in host defense against chitin-containing pathogens,32,33 chitinase-like cytokines are involved primarily in Th2-associated disorders and tumor progression. Chitinase-like proteins are soluble mediators of cell differentiation, proliferation, activation, migration, and adhesion, and can be defined as a novel class of cytokines.34-38 According to its domain organization, lack of the catalytic site, and presence in the macrophage-conditioned medium, SI-CLP can be classified as a novel human Glyco_18 domain–containing chitinase-like secreted protein.
Intracellular localization studies in macrophages revealed that SI-CLP is sorted into Lamp1-positive and secretion-committed CD63+ lysosomes. The lysosomal routing of Glyco_18 domain–containing proteins seems to be different from classic routing (mediated by MPRs) since they lack N-glycosylation typical of lysosomal enzymes.24 Several lines of evidence support the hypothesis that stabilin-1 is a highly specific intracellular sorting receptor for SI-CLP. SI-CLP directly binds stabilin-1, colocalizes with stabilin-1 in TGN in MϕIL-4/Dex, and has impaired lysosomal localization in MϕIL-4/Dex transfected with stabilin-1 siRNA.. Moreover, stabilin-1 mediates relocalization of recombinant SI-CLP-FLAG in H1299 cells, which lack the lysosomal sorting machinery for this protein. Inducible expression of both proteins by the same type of alternatively activated macrophages further supports this notion. The lysosomal localization of SI-CLP in stabilin-1–negative macrophages, however, indicates that stabilin-1 may not be the only receptor mediating trafficking of newly synthesized SI-CLP. The ability of dexamethasone to block secretion of SI-CLP by macrophages additionally confirms that SI-CLP is secreted via a regulated lysosomal pathway.8
Macrophages are the major source of several Glyco_18 domain–containing proteins in mammals. Human chitotriosidase was originally identified as a hallmark of Gaucher disease,39,40 a lysosomal storage disorder characterized by alternative macrophage activation.41 Chitotriosidase is also increasingly produced during the late stages of differentiation of macrophages derived from healthy donors.25,32,40 While YKL-40 was reported to be a marker for the late stage of macrophage differentiation,27 YM1 was found in murine macrophages in healthy tissues42 but also in Th2-polarized immune responses upon challenge with allergen or parasites.28,43-45 AMCase was also shown to be produced by alternatively activated macrophages in a murine model of Th2-associated allergic inflammation.26 Human AMCase was found to be associated with allergic asthma.26 Similarly, we found that SI-CLP is up-regulated in alternatively activated macrophages in vitro upon the combined stimulation by the Th2 cytokine IL-4 and dexamethasone.
The closest SI-CLP homologs YKL-39 and YKL-40 were shown to have diagnostic and prognostic significance. The spectrum of YKL-40–associated disorders correlates with its macrophage origin and biologic activity and includes tumors, infectious diseases,46,47 and disorders characterized by changes in the vascular system and by ECM remodeling such as rheumatoid arthritis,48 inflammatory bowel disease,49 hepatic fibrosis and cirrhosis,50-52 and systemic sclerosis.53 YKL-40 at elevated levels in serum correlates with poor prognosis in patients with ovarian,54 colorectal,55 and breast cancer,56 and serves as a marker for the presence and grade of gliomas.57 Elevated levels of YKL-39 and autoimmune response to YKL-39 are associated with rheumatoid arthritis.58-60 In contrast to YKL-40, YKL-39 has not been reported to be produced by macrophages; this is consistent with our results demonstrating very low levels of YKL-39 transcripts in macrophages. YKL-39 was originally isolated from articular cartilage chondrocyte-conditioned medium,61 and it is significantly up-regulated in osteoarthritic cartilage.62,63 However, neither YKL-40 nor YKL-39 was found by us to be stimulated by glucocorticoids, and in this aspect SI-CLP is a uniquely regulated human chitinase-like protein.
In summary, we have identified a novel human chitinase-like secreted protein interacting with the endocytic/sorting receptor stabilin-1. We demonstrated that stabilin-1 is involved in intracellular routing of SI-CLP. As for stabilin-1, SI-CLP is strongly up-regulated by IL-4 and dexamethasone in alternatively activated macrophages. The ability of rat mAb 1C11 to detect SI-CLP in human PBLs and BALs will allow the investigation of the association of SI-CLP with human disorders.
Prepublished online as Blood First Edition Paper, December 15, 2005; DOI 10.1182/blood-2005-07-2843.
Supported by Margarete von Wrangell Habilitationprogramm (J.K.) and Deutsche Forschungsgemeinschaft SFB405, Project B12.
Three of the authors (J.K., E.K., and S.G.) have applied for an international patent involving mAb 1C11 generation and analysis.
J.K. and S.M. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We gratefully acknowledge the excellent technical assistance of Ms C. Herbst and Ms B. Schleider. We thank Mark Schmidt for the helpful discussion and Mrs Gail Workman for assistance in preparation of the paper.