Abstract
An important aspect of tumor progression is the ability of cancer cells to escape detection and clearance by the immune system. Recent studies suggest that several tumors express soluble factors interfering with the immune response. Here, we show that semaphorin-3A (Sema-3A), a secreted member of the semaphorin family involved in axonal guidance, organogenesis, and angiogenesis, is highly expressed in several tumor cells. Conditioned media of Sema-3A-transfected COS-7 cells or human recombinant Sema-3A inhibited primary human T-cell proliferation and cytokines production under anti-CD3 plus anti-CD28 stimulating conditions. Sema-3A also inhibited the activation of nonspecific cytotoxic activity in mixed lymphocyte culture (MLC), as measured against K-562 cells. In contrast, suppression of Sema-3A in tumor cells with a small interfering RNA (siRNA) augmented T-cell activation. The inhibitory effect of Sema-3A in T cells is mediated by blockade of Ras/mitogen-activated protein kinase (MAPK) signaling pathway. The presence of Sema-3A increased the activation of the Ras family small GTPase Rap1 and introduction of the dominant-negative mutant of Rap1 (Rap1N17) blunted the immunoinhibitory effects of Sema-3A. These results suggest that Sema-3A inhibits primary T-cell activation and imply that it can contribute to the T-cell dysfunction in the tumor microenvironment.
Introduction
The immune system is potentially able to recognize peptide antigens expressed on tumor cells and to mount a protective immune response.1,2 However, tumor cells often develop multiple strategies to evade immune attack.3,4 One of these is represented by the production of soluble immunosuppressive factors that may prevent the proinflammatory effects of tumor-associated antigens, thus promoting T-cell dysfunction in the tumor microenvironment. Accordingly, it has been reported that cancer cells produce several ligands, such as interleukin 10 (IL-10), IL-6, vascular endothelial growth factor (VEGF), TGF-β, and galectin-1, which function to prevent optimal T-cell activation and to induce T-cell anergy or apoptosis.5-8 We investigated here a potential role of semaphorin-3A (Sema-3A) in this function.
The semaphorin family comprises soluble and membrane-bound proteins that function during neuronal development, organogenesis, angiogenesis, and cancer progression.9,10 This family has also attracted the attention of immunologists as novel regulators of immune cell responses.11 Various members of semaphorins act as amplifiers of the immune response,12 whereas others, the secreted semaphorins of class 3 (Sema3) in particular, may negatively control immune functions.13 Moreover, it has become clear that neuropilin and plexin semaphorin receptors are active in the immune system. Neuropilin-1 (NP-1), the receptor of Sema-3A,14 is expressed by T cells and dendritic cells (DCs), and it mediates interaction between DCs and T cells through homotypic interaction.15 Plexins, which transduce semaphorin signals either alone or in a complex with neuropilins,16 are involved in alloantigen and peptide antigen stimulation of T cells.17
Expression of Sema-3A, a prototype member of Sema3, and its receptors has been documented in immune-privileged organs, such as eye18 and testis19 and in some different cancer cells, including those of breast,20 lung,21 glioma,22 and mesothelioma.23 However, little is known about the autocrine/paracrine activity of Sema-3A in these tumors.
Materials and methods
Tumor cells, siRNA transfections, and preparation of tumor supernatants (TSNs)
The tumor cells H28, H2452, MPP89, U87GM, A549, MCF-7, SW480, HCT116, PC3, and LNCaP were previously described.24 The Jurkat leukemic T-cell, HL60 promyelocytic leukemia cell, and Caki-1 renal cancer cell lines were obtained from American Type Culture Collection (Manassas, VA).
Sema-3A small interference RNA (Sema-3A siRNA) was designed, produced, and quality-controlled by the manufacturer (Santa Cruz Biotechnology, Santa Cruz, CA). An identical sequence, with 4 mispairs that were appropriately distributed along the sequence, was used as control (C-siRNA). Treatment of tumor cells with siRNA was done as previously described.25 Two different concentrations (300 and 600 nM) of siRNA were used. After transient transfection, cells were washed and cultured in serum-free containing media for 48 and 72 hours. The TSNs derived from either wild-type or transfected human cancer cells were then centrifuged at 1000g for 5 minutes, concentrated (5 ×) with Centricon (Millipore, Bedford, CA), and collected at -20°C. Equivalent amounts of TSNs (150 μg/sample) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred on nitrocellulose membrane, and analyzed for Sema-3A protein expression by immunoblotting.
Isolation, culture, and T-cell proliferation
Human resting T cells were isolated from the peripheral blood of healthy volunteers, after their informed consent, with the use of pan T-cell isolation kits (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's protocol. Approval was obtained from the Polytechnic University of Marche review board for these studies. The purity of T cells, evaluated by CD4 and CD8 staining, was always higher than 95%. Cells (106 cells/mL) were resuspended in complete RPMI 1640 medium. Anti-CD3 and anti-CD28 (Research Diagnostics, Flanders, NJ) were used at a concentration of 300 and 200 ng/mL, respectively. Human recombinant IL-2 and PMA (Calbiochem, San Diego, CA) were used at 50 U/mL and 5 ng/mL, respectively. T cells were cultured in complete RPMI 1640 medium for 5 to 60 hours at 37°C in 5% CO2 either alone or using anti-CD3 and anti-CD28 in the absence or presence of optimal dilutions of TSNs (1:2) derived from an equal cell number of cancer cells. After indicated times, cultures were pulsed with 0.5 μCi/mL (0.0185 MBq) [3H]-thymidine (Amersham, Milan, Italy) for the last 6 hours. Human recombinant Sema-3A and Sema-6A fused to human Fc fragment (Sema3A/Fc and Sema6A/Fc) were purchased from R&D Systems (Minneapolis, MN). For rescue experiments, human recombinant IL-4 and IL-10 were obtained from PeproTech EC (London, United Kingdom).
Binding of Sema-3A to T cells
T cells (5 × 105 cells/mL) were resuspended in Walsh buffer (137 mM NaCl, 2.7 mM KCl, 1.0 mM MgCl2, 3.3 mM NaH2PO4, 3.8 mM HEPES, 0.1% glucose, 0.1% BSA, pH 7.4) and incubated with different concentrations of Sema3A/Fc for 30 minutes at room temperature. After washing, T cells were incubated in PBS with FITC-labeled anti-human Fc (Sigma, Milan, Italy) for 20 minutes and analyzed by flow cytometry. In some experiments, T cells were first incubated with 10 μg/mL blocking anti-NP-1 antibody or a control Ab for 10 minutes. Then, T cells were incubated with Sema3A/Fc for another 30 minutes, followed by flow cytometry.
Generation of COS-7 clones and serum-free concentrated conditioned media (CM)
The plasmid coding Myc-tagged wild-type Sema-3A was described previously.23 COS-7 cells were cultured at a subconfluence density and transfected using Lipofectamine (Invitrogen, Milan, Italy) for 4 hours. Transfected COS-7 cells were grown for 48 hours and selected, by limiting dilution, with an appropriate drug. More than 10 clones were generated. Representative clones with high (COS-7/C1) or intermediate (COS-7/C2 and COS-7/C3) Sema-3A levels were selected for the experiments. COS-7 cells transfected with vector alone (COS-7/-) were used as control. The CMs obtained from these clones were prepared as described for TSNs. Cell lysates and CMs of transfectants were screened for Sema-3A expression by immunoblotting.
Heterotypic T-cell clustering
T-cell clustering was analyzed as described15 by using wild-type tumor cells or tumor cells that had been transfected with Sema-3A siRNA or C-siRNA. Briefly, T cells were labeled with hydroethidine (40 μg/mL; Molecular Probes, Eugene, OR) for 1 hour at 37°C before mixing with tumor cells (at a ratio of 20:1). The cell mixture was then incubated for 30 minutes at 37°C. The clustering was determined by measuring the percentage of fluorescent T cells that had bound tumor cells by flow cytometry. For the NP-1 functional blocking test, the blocking anti-NP-1 antibody, a generous gift of Dr M. Tessier-Lavigne (Genentech, San Francisco, CA), was previously described.14 The control antibody was a rabbit polyclonal anti-NP-1 antibody (clone H286; Santa Cruz Biotechnology) with a nonblocking function.15 Labeled T cells were preincubated with these 2 antibodies (10 μg/mL) for 30 minutes at 4°C and then mixed with tumor cells.
Real-time RT-PCR
Real-time reverse transcriptase-polymerase chain reaction (RT-PCR) analysis was done in a Chromo4 sequence detector (Bio-Rad, Milan, Italy) as previously described.25 The primers sequences of SEMA3A, SEMA3B, SEMA3F, SEMA3E, NP1, IL2, IL4, IL10, and IFNG genes were determined by Laboratory Tools software analysis of Stratagene (Milan, Italy). Details of sequences and thermal cycle conditions are available on request. The amplicons of genes were between 250 and 300 bp (base pair). Data were acquired and analyzed with the sequence detector Chromo4 software (Bio-Rad, Milan, Italy).
Immunoprecipitation and immunoblot analysis
Immunoprecipitation and immunoblot were performed from whole-cell lysates as previously described.23-26 Antibodies against Sema-3A, NP-1, Raf-1, Ras, Rap-1, c-Jun, phospho-c-Jun, c-Fos, IκBα, phospho-ERK1/2, ERK1, and p38 MAPK (all by Santa Cruz Biotechnology), NFATp, p27kip1 (Transduction Laboratories, Lexington, KY), phospho-MEK1, MEK1, and JNK1 (Cell Signaling Technologies, Milan, Italy), and phospho-p38 MAPK (New England Biolabs, Beverly, MA) were used as probes and detected using enhanced chemiluminescence (Amersham, Milan, Italy). As loading control, blots were reprobed with an antiactin monoclonal antibody (Santa Cruz Biotechnology).
Pulldown assays
Pulldown of Rap1-GTP using a glutathione S-transferase (GST)-RalGDS-Ras binding domain (RBD) fusion protein and Pulldown assays of H-ras-GTP using GST-Raf1-RBD fusion protein were performed with the use of EZ-Detect Rap1 and Ras activation kits (Pierce, Milan, Italy) according to the manufacturer's protocol. Briefly, 107 cells lysed in ice-cold lysis buffer (1% Triton X-100; 50 mM Tris-HCl, pH 7.5; 100 mM NaCl; 10 mM MgCl2; 1 mM phenylmethylsulfonyl fluoride; 1 mM leupeptin; 0.5 mM aprotinin) were incubated with GST-RalGDS-RBD and GST-Raf-RBD fusion proteins coupled to glutathione agarose beads for 1 hour at 4°C. Beads were washed 3 times with lysis buffer and subjected to Western blotting by using anti-Rap1A or anti-H-ras monoclonal antibodies.
Cytokine and growth factors assays
Cytokine and growth factor production was measured and quantified by enzyme-linked immunosorbent assay (ELISA) with the following commercial kits: IFN-γ, IL-2, and IL-4 (Pharmingen, Milan, Italy); and IL-10, IL-6, VEGF, and TGF-β (R&D Systems).
Immunohistochemistry
Immunoperoxidase staining was performed on paraffin-embedded tissue sections from samples of human conventional clear cell renal cell carcinoma using anti-Sema-3A antibody (1:500), anti-NP-1 antibody (1:500), or the same dilution of a control IgG (Santa Cruz Biotechnology). The avidin-biotin peroxidase complex was revealed by using the IHC Select Immunohistochemical Detection System kit (Chemicon, Milan, Italy) according to the manufacturer's instructions. Sections were examined using an Olympus BX60 microscope and a 40 ×/0.85 numeric aperture objective, equipped with an Olympus DP-70 digital camera (Olympus, Milan, Italy). Images were acquired using analySIS image processing software (Soft Imaging System, Münster, Germany).
Jurkat cell transfection and luciferase assays
To evaluate IL-2 transcription, Jurkat cells were electroporated at 960 μF and 250 V using a Bio-Rad Gene Pulser with a constant amount (10 μg) of the reporter construct of luciferase driven by the 2-kb (kilobase) IL-2 promoter-enhancer as described.26 Forty hours after transfection, 5 × 105 cells were divided into aliquots into separate samples and cultured for 6 hours either with anti-CD3 + anti-CD28 alone or with increasing concentrations of Sema3A/Fc, then luciferase activity was measured as described.24 In some experiments, Jurkat cells were transfected with equal amounts (40 μg) of pcDNA1.1/amp empty vector or vector containing Rap1-DN cDNA as previously described.27 The retroviral Babe-puro-Ha-RasV12 and pBabe empty vectors were a generous gift of Dr Geoffrey J. Clark. Infection of cells with retroviral constructs and culturing of transduced cells were performed as described.28 In all experiments the transfection efficiency was normalized by cotransfection with pcDNA1.1-lacZ and assay for β-galactosidase expression.
Cell-cycle analysis
For cell-cycle analysis, cells were permeabilized by adding 0.1% Triton X-100 and incubated with 0.2 mg/mL RNase A (Sigma). Then, T cells were stained with 50 μg/mL propidium iodide (Sigma), and the DNA content was analyzed by flow cytometry as previously described.24
Mixed lymphocyte culture (MLC) assay
Peripheral blood lymphocytes (PBLs) obtained from the blood of recipients were isolated by density gradient centrifugation over Ficoll-Hypaque. PBLs (106/mL) were stimulated with allogenic gamma irradiated (30 Gy [3000 rad]) PBLs at a responder-stimulator ratio of 1:1. Media consisted of RPMI 1640 medium containing 15% normal human serum, 2 mM l-glutamine, 10 mM HEPES, and 100 μg/mL antibiotic-antimycotic solution. After 6 days of incubation, the cells were harvested, washed twice, and used as effector cells in a 4-hour 51Cr release assay that was carried out as previously described.29 The erythroid/myeloid cell line K562 (ATCC) was used as target cells.
Statistical analysis
All values were expressed as mean ± SEM of no less than triplicate measurements of 3 independent experiments. Comparison of results between different groups was performed by 1-way analysis of variance, paired t test, and ANOVA using StatView 5.0 (NET Engineering, Pavia, Italy). A P value of no more than .05 was considered statistically significant.
Results
Sema-3A is expressed by human cancer cells
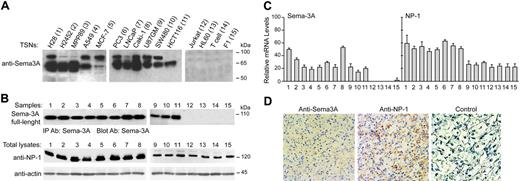
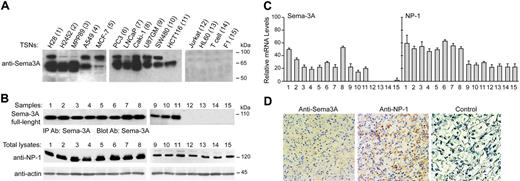
Sema-3A production was assessed in a variety of human tumor cells. In concentrated serum-free TSNs of human mesothelioma (H28, H2452, MPP89), astrocitoma (U87GM), kidney (Caki-1), lung (A549), breast (MCF-7), colorectal (SW480, HCT116), and prostate (PC3 and LNCaP) cancer cells, 2 bands were detected by Western blot analysis: one corresponding to a molecular weight of 95 kDa, representing full-size Sema-3A, and another band of 65 kDa, corresponding to proteolytic-processed Sema-3A (Figure 1A). This was in contrast to TSNs derived from a human T-cell leukemia cell line (Jurkat), promyelocytic leukemia cell line (HL60), resting T cells purified from adult peripheral blood (T cells), and human fibroblasts (F1), which had undetectable Sema-3A protein levels (Figure 1A). In addition, we detected the presence of full-length Sema-3A in immunoprecipitated whole-cell lysates from different tumor cells (Figure 1B). Unlike Jurkat, HL60, T cells, and F1 cells, cancer cells expressed Sema-3A mRNA with a maximum expression observed in H28 and Caki-1 cells (Figure 1C). We also examined the mRNA and protein levels of Sema-3A receptor NP-1. All cell lines, including Jurkat and T cells, expressed NP-1 (Figure 1B-C).
Sema-3A and NP-1 were also expressed in freshly isolated tumors from patients with clear cell renal cell carcinoma, as demonstrated by Western blot (not shown) and immunohistochemistry analysis (Figure 1D). These results confirmed that Sema-3A and NP-1 are coexpressed in human tumor cells in vivo.
Binding of Sema3A to T cells and effects of Sema-3A on T-cell proliferation
It was reported that Sema-3A can inhibit the proliferation of NP-1-expressing cells.9,10,23 We first investigated the binding of human recombinant Sema-3A (Sema3A/Fc) to resting T cells. Sema3A/Fc bound to T cells in a dose-dependent manner and 100 ng/mL Sema3A/Fc saturates the binding to T cells (Figure 2A). This binding is inhibited by the addition of blocking anti-NP-1 antibody (Figure 2A), suggesting that NP-1 is the functional receptor for Sema-3A in T cells.
Expression of Sema-3A and NP-1 in tumor cells and renal cell carcinomas. (A) Western blot detection of secreted Sema-3A in concentrated (5 ×) TSNs (150 μg) from the indicated cells using anti-Sema-3A specific antibody. (B) Total lysates from cell lines indicated in panel A were immunoprecipitated with anti-Sema-3A antibody (IP Ab: Sema-3A). Immune complexes were immunoblotted (Blot Ab) with anti-Sema-3A antibody to detect Sema-3A precursor (top). The same lysates (50 μg) were also processed to detect NP-1 by immunoblotting. Expression of actin was used as internal control. Data are representative of 3 experiments. Sizes are shown in kilodaltons. (C) Total RNA (50 ng/μL) was isolated from the indicated cells and subjected to real-time PCR, as described in “Materials and methods,” to detect SEMA3A and NP1 transcripts. The mRNA levels of each gene were normalized by the mRNA levels of housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Reported values are an average of 3 separate samples of each type analyzed in triplicate; bars, ± SE. (D) Immunoperoxidase staining of a paraffin-embedded tissue section of a representative human renal cell carcinoma specimen (Clear cell type, grade 3, stage I) using an anti-Sema-3A and anti-NP-1 antibody or the same dilution of a control IgG (magnification, × 40). Scarce amounts of Sema-3A and NP-1 were also detected in normal renal tissue (data not shown). Antibody localization was effected using a peroxidase reaction with 3,3-diaminobenzidine tetrahydrochloride as chromogen.
Expression of Sema-3A and NP-1 in tumor cells and renal cell carcinomas. (A) Western blot detection of secreted Sema-3A in concentrated (5 ×) TSNs (150 μg) from the indicated cells using anti-Sema-3A specific antibody. (B) Total lysates from cell lines indicated in panel A were immunoprecipitated with anti-Sema-3A antibody (IP Ab: Sema-3A). Immune complexes were immunoblotted (Blot Ab) with anti-Sema-3A antibody to detect Sema-3A precursor (top). The same lysates (50 μg) were also processed to detect NP-1 by immunoblotting. Expression of actin was used as internal control. Data are representative of 3 experiments. Sizes are shown in kilodaltons. (C) Total RNA (50 ng/μL) was isolated from the indicated cells and subjected to real-time PCR, as described in “Materials and methods,” to detect SEMA3A and NP1 transcripts. The mRNA levels of each gene were normalized by the mRNA levels of housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Reported values are an average of 3 separate samples of each type analyzed in triplicate; bars, ± SE. (D) Immunoperoxidase staining of a paraffin-embedded tissue section of a representative human renal cell carcinoma specimen (Clear cell type, grade 3, stage I) using an anti-Sema-3A and anti-NP-1 antibody or the same dilution of a control IgG (magnification, × 40). Scarce amounts of Sema-3A and NP-1 were also detected in normal renal tissue (data not shown). Antibody localization was effected using a peroxidase reaction with 3,3-diaminobenzidine tetrahydrochloride as chromogen.
Sema-3A binds T cells and inhibits T-cell proliferation. (A) Resting T cells were incubated with Sema3A/Fc or hIgG and then analyzed by flow cytometry as described in “Materials and methods.” Mean fluorescence intensity (MFI) derived from 3 independent experiments is shown (left). Alternatively, cells were first incubated with the blocking anti-NP-1 antibody or a control Ab. Then, T cells were treated with Sema3A/Fc or hIgG as described (right). (B) COS-7 cells were stably transfected with a vector that allowed a high (COS-7/C1) and intermediate (COS-7/C2 and COS-7/C3) amount of Sema-3A expression, as shown by Western blot in concentrated serum-free supernatants (CM) (insert). CM (48 hours of conditioning, 1:2 of dilution) derived from COS-7 cells and Sema-3A-expressing COS-7 cells were incubated with T cells for 18 hours in the absence (Control) or in the presence of soluble anti-CD3 (300 ng/mL), either alone or with anti-CD28 (200 ng/mL) or PMA (5 ng/mL). T-cell proliferation was then measured by [3H]thymidine incorporation. *P ≤ .001 versus COS-7/-, ANOVA, n = 3. (C) T cells were treated with CM derived form COS-7/- and COS-7/C1 clones, stained with PI and analyzed by flow cytometry. (D) T cells were treated as described in panel A in the presence of CM derived form COS-7/- and COS-7/C1 clones for the indicated times, and the p21Kip1 protein expression was detected by Western blotting with anti-p27Kip1 antibody. Expression of actin was used as loading control. Data are representative of 3 experiments. Error bars indicate SEM (n = 3).
Sema-3A binds T cells and inhibits T-cell proliferation. (A) Resting T cells were incubated with Sema3A/Fc or hIgG and then analyzed by flow cytometry as described in “Materials and methods.” Mean fluorescence intensity (MFI) derived from 3 independent experiments is shown (left). Alternatively, cells were first incubated with the blocking anti-NP-1 antibody or a control Ab. Then, T cells were treated with Sema3A/Fc or hIgG as described (right). (B) COS-7 cells were stably transfected with a vector that allowed a high (COS-7/C1) and intermediate (COS-7/C2 and COS-7/C3) amount of Sema-3A expression, as shown by Western blot in concentrated serum-free supernatants (CM) (insert). CM (48 hours of conditioning, 1:2 of dilution) derived from COS-7 cells and Sema-3A-expressing COS-7 cells were incubated with T cells for 18 hours in the absence (Control) or in the presence of soluble anti-CD3 (300 ng/mL), either alone or with anti-CD28 (200 ng/mL) or PMA (5 ng/mL). T-cell proliferation was then measured by [3H]thymidine incorporation. *P ≤ .001 versus COS-7/-, ANOVA, n = 3. (C) T cells were treated with CM derived form COS-7/- and COS-7/C1 clones, stained with PI and analyzed by flow cytometry. (D) T cells were treated as described in panel A in the presence of CM derived form COS-7/- and COS-7/C1 clones for the indicated times, and the p21Kip1 protein expression was detected by Western blotting with anti-p27Kip1 antibody. Expression of actin was used as loading control. Data are representative of 3 experiments. Error bars indicate SEM (n = 3).
Next, we transfected COS-7 cells with a Sema-3A expression vector to generate different clones releasing low or intermediate Sema-3A levels in the conditioned medium (CM). Western blot analysis of CM collected from clone 1 (COS-7/C1) demonstrated the highest levels of secreted Sema-3A compared with cells transfected with empty vector (COS-7/-), whereas clones 2 and 3 (COS-7/C2 and COS-7/C3) secreted intermediate levels of Sema-3A (35% and 69% relative to clone 1, respectively) (Figure 2B, insert). We tested the ability of Sema-3A containing CMs to inhibit anti-CD3 and anti-CD28-induced T-cell proliferation. The amounts of Sema-3A in CMs directly correlate with the inhibition of T-cell proliferation (Figure 2B). Sema-3A containing CMs had no effect on T-cell proliferation induced by phorbol-12-myristate-13-acetate (PMA) (Figure 2B). Similar effects to CM obtained from Sema-3A-expressing clones were observed when using Sema3A/Fc (IC50 ∼ 55 ng/mL). By contrast, Sema6A/Fc (up to 100 ng/mL), a semaphorin that cannot bind to NP-1, was ineffective (Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article).
In the presence of CM derived from COS-7/C1 clones, CD3 plus CD28-mediated T-cell growth was clearly delayed, with an increase in the fraction in G0/G1 phase, a proportional reduction in S and G2/M phase, and no detectable sub-G1 fraction (Figure 2C). This treatment increased expression of p27kip1, a cyclin-CDK inhibitor that is a hallmark of the G1 phase accumulation (Figure 2D). By contrast, the treatment with CM derived from COS-7/- cells did not affect cell distribution in cell-cycle phases and p27kip1 expression (Figure 2C-D). Treatment with Sema3A/Fc had analogous effects (data not shown). Thus, Sema-3A inhibited T-cell proliferation by promoting the synthesis of a negative regulator of the cell cycle, such as p27kip1.
Effects of Sema-3A-containing CMs on cytokine transcription and cytotoxic activities generated in MLC
We analyzed the functional effect of Sema-3A on cytokine production by T cells. CM collected from COS-7/C1 and COS-7/C2 cells inhibited the production of IL-2, interferon-γ (INF-γ), IL-4, and IL-10 induced by anti-CD3 and -CD28 costimulation (Figure 3A). Both COS-7/C1- and COS-7/C2-derived CM also inhibited mRNA expression of these cytokines as determined by quantitative real-time PCR (data not shown). In particular, they induced a significant reduction of IL-2 mRNA (Figure 3B). Notably, the addition of exogenous IL-2 reversed the inhibitory effect of COS-7/C1-derived CM on T-cell proliferation, whereas equimolar concentrations of IL-4 and IL-10 were ineffective (Figure 3C). We also assessed the effects of Sema-3A-containing CMs on the generation of cytotoxic activity from human PBLs in MLC. Unlike IL-2, CM collected from COS-7/C1 and COS-7/C2 cells inhibited the generation of cytolytic activity against K562 cells (Table 1). The recoveries of the cultures in Sema-3A-containing medium were comparable and only slightly higher (10%-15%) than those of the MLC cultured in medium only (data not shown).
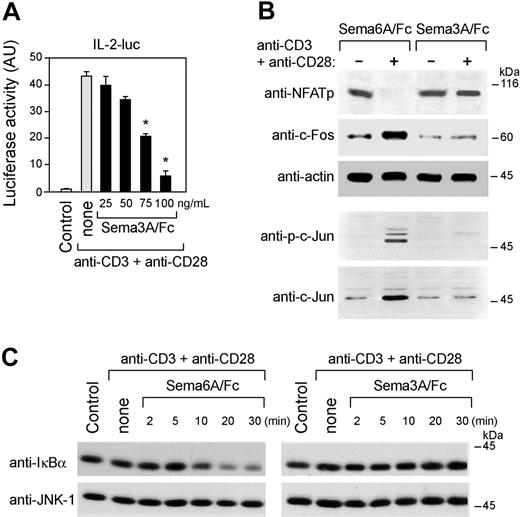
To analyze the mechanism(s) by which Sema-3A inhibited IL-2 production, we first transfected Jurkat cells with a reporter luciferase construct coding for the 2-kb IL-2 promoter. Treatment of these Jurkat cells with Sema3A/Fc resulted in dose-dependent inhibition of IL-2 gene transcription in response to anti-CD3 and anti-CD28 costimulation (Figure 4A). Sema3A/Fc affected the induction or phosphorylation of c-Jun, c-Fos and the dephosphorylation of NFATp (Figure 4B). Although Sema6A/Fc was ineffective, Sema3A/Fc also blocked the degradation of IκBα induced by anti-CD3 and anti-CD28 costimulation, a necessary prerequisite for the activation of NF-κB (Figure 4C). These data suggest that the negative regulation of IL-2 transcription by Sema-3A implicates a defective expression or transactivation of known IL-2 transcription factors.
Knockdown of Sema-3A in tumor cells increases T-cell activation
TSNs contain several soluble immunosuppressive factors.5-8 In addition, other secreted semaphorins, such as Sema-3B, -3F, and -3E, are involved in oncogenesis.10 Sema-3B and Sema-3F were produced at very low levels by tumor cells examined and were not produced in Caki-1 and H28 cells. By contrast, detectable amounts of Sema-3E mRNA were identified in these cells (data not shown). We reduced Sema-3A expression in tumor cells with a targeted small interfering RNA (Sema-3A siRNA). After 48 hours following transfection, Sema-3A siRNA, but not a control siRNA (C-siRNA), reduced mRNA levels of Sema-3A in both Caki-1 and H28 cells. Notably, Sema-3E mRNA levels were unchanged (Figure S2). Either immunoprecipitated whole-cell lysates or TSNs collected from Caki-1 and H28 cells exposed for 72 hours to Sema-3A siRNA revealed a strong inhibition of Sema-3A production and secretion (Figure 5A). Both Sema-3A siRNA and C-siRNA did not alter cell growth rate (data not shown) or protein expression of IL-6, VEGF, and TGF-β in H28 cells (Figure 5B), which function as immunosuppressive factors secreted by tumor cells.
Sema-3A reduces cytokines production. (A) T cells were cultured for the indicated times with anti-CD3 (300 ng/mL) plus anti-CD28 (200 ng/mL) alone or with CM derived from Sema-3A-expressing COS-7 cells as described in Figure 2A. Supernatants were collected, and IL-2, IL-10, IFN-γ, and IL-4 concentrations were assessed by ELISA. (B) T cells were treated as described in panel A for 8 hours. Total RNA was extracted, and IL-2 expression was analyzed by real-time PCR. GAPDH were used as housekeeping gene. *P < .001 versus COS-7/-, ANOVA, n = 3. (C) T cells were cultured for 24 hours with anti-CD3 + anti-CD28 in the presence of CM derived from COS-7/- or COS-7/C1 clones. Recombinant human IL-2, IL-4, and IL-10 (1.0 ng/mL) were added to the culture, and T-cell proliferation was measured by [3H]thymidine incorporation. *P < .05 versus untreated cells, paired t test, n = 3. Error bars indicate SEM.
Sema-3A reduces cytokines production. (A) T cells were cultured for the indicated times with anti-CD3 (300 ng/mL) plus anti-CD28 (200 ng/mL) alone or with CM derived from Sema-3A-expressing COS-7 cells as described in Figure 2A. Supernatants were collected, and IL-2, IL-10, IFN-γ, and IL-4 concentrations were assessed by ELISA. (B) T cells were treated as described in panel A for 8 hours. Total RNA was extracted, and IL-2 expression was analyzed by real-time PCR. GAPDH were used as housekeeping gene. *P < .001 versus COS-7/-, ANOVA, n = 3. (C) T cells were cultured for 24 hours with anti-CD3 + anti-CD28 in the presence of CM derived from COS-7/- or COS-7/C1 clones. Recombinant human IL-2, IL-4, and IL-10 (1.0 ng/mL) were added to the culture, and T-cell proliferation was measured by [3H]thymidine incorporation. *P < .05 versus untreated cells, paired t test, n = 3. Error bars indicate SEM.
We tested whether TSNs obtained from Sema-3A knockdown transfectants altered the ability of T cells to respond to anti-CD3 and anti-CD28 costimulation. Unlike TSNs obtained from Caki-1 cells transfected with C-siRNA, TSNs derived from tumor cells transfected with Sema-3A siRNA augmented the proliferative response and cytokine production of T cells (Figure 5C-D). Notably, addition of Sema3A/Fc, but not of Sema6A/Fc, blunted these effects (Figure 5C-D). Similar results were observed when we used TSNs derived from H28 cells instead of Caki-1 cells (data not shown). These results suggested that one mechanism by which tumor cells inhibit T-cell activation may be Sema-3A expression.
Sema-3A inhibits tumor-T-cell interactions and CD3/CD28-mediated activation of Ras/MAPK pathway
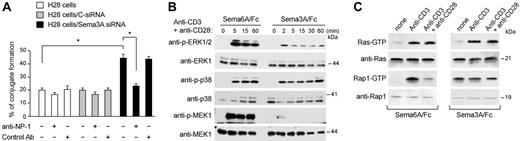
Because NP-1 mediates DC-T-cell clustering,15 we analyzed whether the knockdown of Sema-3A in tumor cells increased the recruitment of resting T cells. Compared with H28 cells transfected with C-siRNA, T cells were weakly but significantly induced (up to 50% increase) to form clusters around H28 cells transfected with Sema-3A siRNA (Figure 6A). This effect was NP-1 dependent, because the addition of blocking anti-NP-1 antibody prevented cluster formation (Figure 6A). Therefore, tumor-derived Sema-3A can inhibit T-cell adhesion to tumor cells in a NP-1-dependent manner.
Sema-3A inhibited CD3 plus CD28-mediated IL-2 transcription and expression and transactivation of known IL-2 transcription factors. (A) Jurkat T cells were transiently transfected with the reporter construct pIL-2-luc (10 μg). Forty hours later, 5 × 105 cells per sample were cultured for 8 hours with either anti-CD3 + anti-CD28 alone or with increasing concentrations of recombinant human Sema-3A (Sema3A/Fc), and luciferase activity was measured. *P < .05 versus none, ANOVA, n = 4. Error bars indicate SEM. (B) Jurkat cells were cultured for 3 hours with anti-CD3 + anti-CD28 in the presence of Sema3A/Fc or Sema6A/Fc (100 ng/mL), whole-cell extracts were prepared, and expression of transcription factors was determined by immunoblotting with antibodies specific for NFATp, c-Fos, and actin. The whole-cell extracts was also immunoblotted using anti-phospho-c-Jun and anti-c-Jun antibodies. (C) Whole-cell extracts from Jurkat cells, treated as described in panel B for the indicated times, were prepared and immunoblotted with an anti-IκBα antibody and reprobed with an anti-JNK1 antibody to control for loading. Data are representative of 2 experiments.
Sema-3A inhibited CD3 plus CD28-mediated IL-2 transcription and expression and transactivation of known IL-2 transcription factors. (A) Jurkat T cells were transiently transfected with the reporter construct pIL-2-luc (10 μg). Forty hours later, 5 × 105 cells per sample were cultured for 8 hours with either anti-CD3 + anti-CD28 alone or with increasing concentrations of recombinant human Sema-3A (Sema3A/Fc), and luciferase activity was measured. *P < .05 versus none, ANOVA, n = 4. Error bars indicate SEM. (B) Jurkat cells were cultured for 3 hours with anti-CD3 + anti-CD28 in the presence of Sema3A/Fc or Sema6A/Fc (100 ng/mL), whole-cell extracts were prepared, and expression of transcription factors was determined by immunoblotting with antibodies specific for NFATp, c-Fos, and actin. The whole-cell extracts was also immunoblotted using anti-phospho-c-Jun and anti-c-Jun antibodies. (C) Whole-cell extracts from Jurkat cells, treated as described in panel B for the indicated times, were prepared and immunoblotted with an anti-IκBα antibody and reprobed with an anti-JNK1 antibody to control for loading. Data are representative of 2 experiments.
Down-regulation of Sema-3A expression in tumor cells sensitizes T-cell activation. (A) Caki-1 and H28 cells were transfected with C-siRNA or Sema-3A siRNA (600 nM). Then, immunoprecipitated whole-cell lysates and TSNs were prepared 72 hours after transfection and analyzed as reported in Figure 1. (B) TSNs of transfected H28 cells were collected, and IL-6, TGF-β, and VEGF concentrations were assessed by ELISA. (C-D) T cells were cultured for 24 hours with either anti-CD3 + anti-CD28 alone or with the indicated TSNs (1:2) from Caki-1 cells in the absence or presence of increasing concentrations of Sema3A/Fc or Sema6A/Fc. T-cell proliferation was then assessed by [3H]-thymidine uptake (C). In parallel, supernatants of T cells were collected, and IL-2 and IFN-γ concentrations were assessed by ELISA (D). *P < .001, ANOVA, n = 4. Error bars indicate SEM.
Down-regulation of Sema-3A expression in tumor cells sensitizes T-cell activation. (A) Caki-1 and H28 cells were transfected with C-siRNA or Sema-3A siRNA (600 nM). Then, immunoprecipitated whole-cell lysates and TSNs were prepared 72 hours after transfection and analyzed as reported in Figure 1. (B) TSNs of transfected H28 cells were collected, and IL-6, TGF-β, and VEGF concentrations were assessed by ELISA. (C-D) T cells were cultured for 24 hours with either anti-CD3 + anti-CD28 alone or with the indicated TSNs (1:2) from Caki-1 cells in the absence or presence of increasing concentrations of Sema3A/Fc or Sema6A/Fc. T-cell proliferation was then assessed by [3H]-thymidine uptake (C). In parallel, supernatants of T cells were collected, and IL-2 and IFN-γ concentrations were assessed by ELISA (D). *P < .001, ANOVA, n = 4. Error bars indicate SEM.
NP-1 has no intrinsic kinase activity but is associated with several coreceptors such as plexins, VEGF receptors, and integrins.10 It probably uses these to mediate a Sema-3A signaling that interferes with other pathways. We examined the activation of extracellular signal-regulated kinase (ERK)1/2, a downstream signaling molecule in the Ras pathway essential during the interaction of T cell with target cell.30 Sema3A/Fc inhibited CD3 plus CD28-mediated ERK1/2 activation in T cells (Figure 6B, top). The inhibition occurred 2 to 5 minutes after the addition of Sema3A/Fc and persisted for at least 60 minutes. The inhibitory effects seemed to be specific to ERK1/2, because p38 MAPK activity was not affected (Figure 6B, middle). We also determined the effects of Sema-3A on MEK, an upstream kinase of ERK1/2. Sema3A/Fc, but not Sema6A/Fc, inhibited MEK activation induced by anti-CD3 and anti-CD28 costimulation (Figure 6B, bottom). Similar results were obtained using Jurkat cells instead to primary T cells (data not shown).
We then analyzed the activation status of Ras in Jurkat cells. Sema3A/Fc did not significantly inhibited the activation of Ras induced by CD3 alone or CD3 plus CD28 (Figure 6C, top). However, when we infected Jurkat cells with constitutively activated Ras (Ha-RasV12), ERK1/2 activity was not inhibited by Sema3A/Fc (Figure S3A). Ha-RasV12 also rendered Jurkat cells refractory to growth inhibition by Sema3A/Fc (Figure S3B). NP-1 was expressed at similar levels and exhibited a similar distribution in infected-Jurkat cells (data not shown), indicating that the loss of responsiveness to Sema-3A was not due to altered NP-1 expression and/or distribution. Therefore, Sema-3A attenuated the CD3 plus CD28-mediated activation of Ras/MAPK pathway and constitutively activated Ras bypassed the responsiveness to Sema-3A.
Rap1 mediates the immunoinhibitory effects of Sema-3A
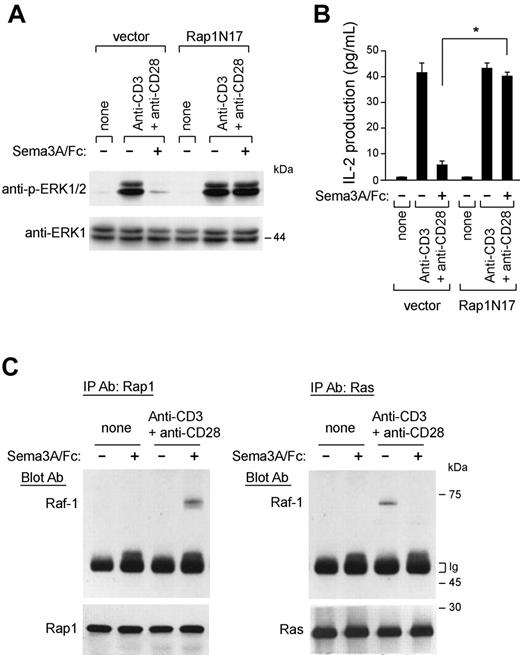
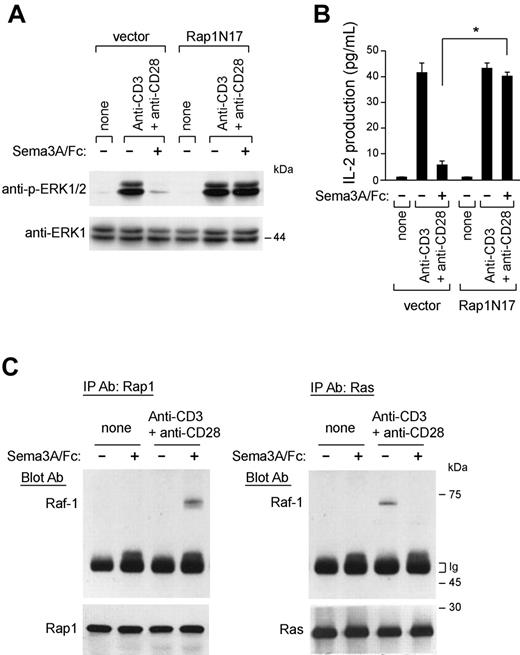
It has been previously demonstrated that the small G protein Rap1 antagonizes Ras/MAPK signaling and negatively regulates IL-2 production.26 Interestingly, we found that active Rap1-GTP levels increased within minutes on Sema3A/Fc stimulation (Figure 6C, bottom) and were sustained for as long as 30 minutes (data not shown). Sema3A/Fc also prevents the reported decrease of Rap1-GTP levels triggered by anti-CD3 and anti-CD28 costimulation31 (Figure 6C, bottom). To test the functional relevance of Rap1 in Sema-3A-mediated inhibition of Ras/Raf/MEK/MAPK signaling and IL-2 transcription, we expressed a dominant-negative Rap1 (Rap1N17) in Jurkat cells and found that this was sufficient to abrogate both effects induced by Sema3A/Fc (Figure 7A-B).
Sema-3A signaling. (A) H28 cells were transfected as described in Figure 5. Seventy-two hours from transfection, cells were incubated with T cells that had been prelabeled with the fluorescent dye hydroethidine. Heterotypic cell clustering was measured by flow cytometry. The involvement of NP-1 in heterotypic cell clustering was studied by preincubating labeled allogenic resting T cells with blocking NP-1 antibodies (T cells + anti-NP-1) or with nonblocking NP-1 antibody (T cells + Control Ab). *P < .05, ANOVA, n = 3. Error bars indicate SEM. (B) T cells were stimulated for the indicated time points with anti-CD3 + anti-CD28 in the presence of Sema3A/Fc or Sema6A/Fc (100 ng/mL). Cell lysates were then immunoblotted with antibodies against phosphorylated ERK1/2 (anti-p-ERK1/2), p38 MAPK (anti-p-38), or phosphorylated MEK1 (anti-p-MEK1). The blots were stripped and reprobed for total ERK1, p38, or MEK1. (C) Jurkat cells were stimulated with anti-CD3 alone or with anti-CD28 in the presence of Sema3A/Fc (right) or Sema6A/Fc (left) (100 ng/mL) for 10 minutes and lysed. GTP-bound Rap1 and H-ras were detected with pulldown assays by using immobilized GST fusion proteins of RalGDS-RBD and Raf-RBD as described in “Materials and methods.” Western blot of total cell lysates with anti-Ras and anti-Rap1 antibody is also shown. Data are representative of 3 experiments.
Sema-3A signaling. (A) H28 cells were transfected as described in Figure 5. Seventy-two hours from transfection, cells were incubated with T cells that had been prelabeled with the fluorescent dye hydroethidine. Heterotypic cell clustering was measured by flow cytometry. The involvement of NP-1 in heterotypic cell clustering was studied by preincubating labeled allogenic resting T cells with blocking NP-1 antibodies (T cells + anti-NP-1) or with nonblocking NP-1 antibody (T cells + Control Ab). *P < .05, ANOVA, n = 3. Error bars indicate SEM. (B) T cells were stimulated for the indicated time points with anti-CD3 + anti-CD28 in the presence of Sema3A/Fc or Sema6A/Fc (100 ng/mL). Cell lysates were then immunoblotted with antibodies against phosphorylated ERK1/2 (anti-p-ERK1/2), p38 MAPK (anti-p-38), or phosphorylated MEK1 (anti-p-MEK1). The blots were stripped and reprobed for total ERK1, p38, or MEK1. (C) Jurkat cells were stimulated with anti-CD3 alone or with anti-CD28 in the presence of Sema3A/Fc (right) or Sema6A/Fc (left) (100 ng/mL) for 10 minutes and lysed. GTP-bound Rap1 and H-ras were detected with pulldown assays by using immobilized GST fusion proteins of RalGDS-RBD and Raf-RBD as described in “Materials and methods.” Western blot of total cell lysates with anti-Ras and anti-Rap1 antibody is also shown. Data are representative of 3 experiments.
Rap1 mediates the immunoinhibitory effects of Sema-3A. (A-B) Jurkat T cells were transfected with an empty vector (pcDNA3.1) or a vector containing cDNA of a dominant-negative Rap1 (Rap1N17). Then, stable transfectants were stimulated with anti-CD3 + anti-CD28 in the absence or presence of Sema3A/Fc (100 ng/mL) for 10 minutes and lysed. Cell lysates were electrophoresed and blotted with anti-p-ERK1/2 antibody. The blots were stripped and reprobed for total ERK1 (A). After 24 hours, the supernatants were harvested for IL-2 measurement by ELISA. *P ≤ .05, ANOVA, n = 3. Error bars indicate SEM. (C) Control and anti-CD3 and anti-CD28-stimulated T cells (107 cells per test) were either untreated or treated with Sema3A/Fc (100 ng/mL) for 5 minutes, and the lysates were then prepared. Equivalent amounts of whole-cell lysates were either immunoprecipitated with anti-Ras antibody and immunoblotted with anti-Raf-1 antibody (right) or immunoprecipitated with anti-Rap-1 antibody and immunoblotted with anti-Raf-1 antibody (left). Western blot of immunoprecipitated cell lysates with anti-Rap1 and anti-Ras antibody is also shown. Data are representative of 3 experiments.
Rap1 mediates the immunoinhibitory effects of Sema-3A. (A-B) Jurkat T cells were transfected with an empty vector (pcDNA3.1) or a vector containing cDNA of a dominant-negative Rap1 (Rap1N17). Then, stable transfectants were stimulated with anti-CD3 + anti-CD28 in the absence or presence of Sema3A/Fc (100 ng/mL) for 10 minutes and lysed. Cell lysates were electrophoresed and blotted with anti-p-ERK1/2 antibody. The blots were stripped and reprobed for total ERK1 (A). After 24 hours, the supernatants were harvested for IL-2 measurement by ELISA. *P ≤ .05, ANOVA, n = 3. Error bars indicate SEM. (C) Control and anti-CD3 and anti-CD28-stimulated T cells (107 cells per test) were either untreated or treated with Sema3A/Fc (100 ng/mL) for 5 minutes, and the lysates were then prepared. Equivalent amounts of whole-cell lysates were either immunoprecipitated with anti-Ras antibody and immunoblotted with anti-Raf-1 antibody (right) or immunoprecipitated with anti-Rap-1 antibody and immunoblotted with anti-Raf-1 antibody (left). Western blot of immunoprecipitated cell lysates with anti-Rap1 and anti-Ras antibody is also shown. Data are representative of 3 experiments.
The antagonism of active Rap1 to ERKs may be due to sequestration of active Raf-1.26,32 Sema3A/Fc strongly increased the association of Raf-1 and Rap1 in T cells, whereas in turn it reduced the association of Raf-1 with Ras, after anti-CD3 and anti-CD28 costimulation (Figure 7C). These results support the idea that Sema-3A antagonizes Ras/MAPK signaling in T cells by inducing Rap1 activation.
Discussion
In this study, we demonstrated that a signal secreted by cancer cells, Sema-3A, acts in a paracrine manner in the tumor microenvironment to inhibit the proliferation of recruited T lymphocytes and their production of immune cytokines. We further demonstrated that Sema-3A specifically increases the levels of Rap1-GTP, a negative regulator of TCR-mediated IL-2 transcription.26 Together with data indicating that increased Rap1 activity reduces Ras/MAPK signals and IL-2 production with accumulation of p27Kip1 in T cells,27 Rap1 induction by Sema-3A provides a mechanism to explain its ability to antagonize the Ras/MAPK pathway and to regulate T-cell functions.
Given the key role of IL-2 to induce T-cell proliferation and differentiation, an important consideration of the present study concerns the potential effects of Sema-3A on cytotoxic T lymphocyte (CTL) generation and/or the activity of already committed CTLs. We found that supernatants derived from Sema-3A expressing COS-7 clones inhibited the activation of nonspecific cytotoxic activity in MLC, as measured against K-562 cells (Table 1). However, our preliminary results do not reveal significant differences in the cytotoxic activities against the specific stimulator cells of the isolated CTL clones. These findings, along with the observations reported in the present study, may suggest that Sema-3A affects the generation of CTL activity in MLCs. This point is currently under investigation.
How Sema-3A can affect T-cell activation is presently unknown. On binding to target cells, a specific large-scale molecular complex is built at target cell-T-cell interface that establishes a specialized junction, namely immunologic synapse.33 The formation of this highly specialized tight interaction is a multistep process that begins with adhesion between these cells.34 NP-1 is involved in initial cell-cell contact between T cells and antigen-presenting cells, probably because of the homophilic interactions.15 Both tumor and T cells expressed NP-1, and the cluster formation between tumor and T cells increased when we reduced Sema-3A expression in tumor cells. This effect was rescued by adding a blocking anti-NP-1 antibody (Figure 6A). Therefore, tumor-derived Sema-3A may competitively inhibit T-cell adhesion to tumor cells by perturbing NP-1-NP-1 homotypic interaction.
It may be postulated a role of the semaphorin-coreceptors plexins in the immunoregulatory activity of Sema-3A. Indeed, the intracellular domain of NP-1 is short, and apparently unable to mediate functional responses to Sema-3A.9 Intriguingly, Plexin-A1 is a coreceptor for Sema-3A in the nervous system,16 and it is involved in the interaction between T cells and DCs17 ; however, we did not detect plexin-A1 mRNA in T cells by Northern blot analysis, whereas it was widely expressed in cancer cells (A.C., unpublished data, May 2005). Therefore, Sema-3A may act through other members of the plexin A subfamily in T cells. Moreover, the receptor for Sema-3A could involve additional components, as described for other family members.10
In this study, we focused on the regulatory role of Sema-3A on Ras/Raf-1/MEK/ERK1/2 pathway, which is indispensable for cytokine production and cell-cycle progression of T lymphocytes.27,30 Our results showed that Sema-3A inhibits CD3/CD28-mediated activation of MEK/ERK1/2 signaling, resulting in impaired cytokine secretion and cell-cycle progression. These events were secondary to defective CD3 plus CD28-mediated activation of Ras and were not observed when proximal TCR signals were bypassed by PMA, which activates Ras, or by the use of a constitutively activated Ras. We found that Sema-3A increased the levels of Rap1-GTP and that introduction of the Rap1N17 blocked the inhibitory effects of Sema-3A on ERK1/2 and IL-2 production. It has been proposed that the antagonism of signals to ERKs by Rap1 may be due to the sequestration of activated Raf-1 by activated Rap1.32 Consistent with this model and as previously observed,31 CD3 stimulation of Jurkat cells activated Ras and Raf-1 as well as Rap1 (Figure 6C) promoting the association of Raf-1 with Rap1 as well as Raf-1 with Ras. By contrast, Sema-3A-dependent pathway activated Rap1, whereas it had no effect on Ras-GTP (Figure 6C, top) and, probably, Raf-1-GTP. Therefore, the association of Rap1 and Raf-1 was not observed in the presence of Sema-3A alone (Figure 7C), presumably because Rap1 sequesters Raf-1 only in the GTP-loaded state. Rap1 activation is not only essential to regulate the functional coupling of T cells with antigen-presenting cells but it can profoundly affect the subsequent course of the T-cell response.27 Thus, regulatory mechanisms implicating Rap1 activation/inactivation may be an important target to overcome T-cell dysfunction in the tumor microenvironment. Our data indicate a novel role of Sema-3A in the interaction between T cells and target tumor cells, by fine-tuning Rap1 activation in lymphocytes to curb Ras/MAPK pathway and effective immunologic responses.
NP-1 expression within tumors has been described35 and often correlated with tumor progression.36,37 Previously, we and others had reported Sema-3A expression in malignant cells.20-23 Here, we showed that a number of tumor cells as well as primary clear cell renal cell carcinomas express Sema-3A and NP-1. Studies suggest that Sema-3A may regulate tumor progression (eg, affecting angiogenesis,38 monocyte migration,12 and tumor cell motility20 ). However, because human tumors may express additional semaphorins that can antagonize Sema-3A-mediated effects,10 future studies are warranted to elucidate the effect of Sema-3A on tumor progression.
In some cancer cells, such as HCT116, we identified a proteolytic-processed form of Sema-3A corresponding to a band of about 65 kDa, which was previously described as a partially inactivated form of Sema-3A generated by furinlike endoproteases.39 However, HCT116 cell supernatants also reduced CD3/CD28-induced T-cell proliferation (S.M., unpublished data, September 2005). This suggests that both forms of Sema-3A may have a similar inhibitory effect on T-cell activation.
NP-1 is also expressed in immune cells and endothelial cells.15 Thus, Sema-3A may act on 2 levels during cancer progression: on tumor cells and on a variety of nontumor cells that are found in the tumor microenvironment. The data presented here indicate, for the first time, a potentially important function for Sema-3A as a paracrine negative regulator of T-cell activation, released by tumor cells. They also provide a link between the immunoregulatory properties of this protein and Rap1 activity. Therefore, our data highlight a novel molecular target for the manipulation of T-cell-dependent immunity with important implications for cancer immunotherapy.
Prepublished online as Blood First Edition Paper, December 27, 2005; DOI 10.1182/blood-2005-06-2445.
Supported by grants from the Italian Association for Cancer Research (AIRC) (A.P. and A.C.); the Ministry of University and the Ministry of Health (A.P.); and by a fellowship from AIRC (S.M.).
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Geoffrey J. Clark (National Cancer Institute, National Institutes of Health, MD) for providing pBabe empty and pBabe HaRasV12 vectors, Dr M. Tessier-Lavigne (Howard Hughes Medical Institute, Stanford University, CA) for providing the blocking anti-NP-1 antibody, and Dr M. R. Rippo and Dr G. Biasi (Polytechnic University of Marche, Ancona, Italy) for discussions.

![Figure 2. Sema-3A binds T cells and inhibits T-cell proliferation. (A) Resting T cells were incubated with Sema3A/Fc or hIgG and then analyzed by flow cytometry as described in “Materials and methods.” Mean fluorescence intensity (MFI) derived from 3 independent experiments is shown (left). Alternatively, cells were first incubated with the blocking anti-NP-1 antibody or a control Ab. Then, T cells were treated with Sema3A/Fc or hIgG as described (right). (B) COS-7 cells were stably transfected with a vector that allowed a high (COS-7/C1) and intermediate (COS-7/C2 and COS-7/C3) amount of Sema-3A expression, as shown by Western blot in concentrated serum-free supernatants (CM) (insert). CM (48 hours of conditioning, 1:2 of dilution) derived from COS-7 cells and Sema-3A-expressing COS-7 cells were incubated with T cells for 18 hours in the absence (Control) or in the presence of soluble anti-CD3 (300 ng/mL), either alone or with anti-CD28 (200 ng/mL) or PMA (5 ng/mL). T-cell proliferation was then measured by [3H]thymidine incorporation. *P ≤ .001 versus COS-7/-, ANOVA, n = 3. (C) T cells were treated with CM derived form COS-7/- and COS-7/C1 clones, stained with PI and analyzed by flow cytometry. (D) T cells were treated as described in panel A in the presence of CM derived form COS-7/- and COS-7/C1 clones for the indicated times, and the p21Kip1 protein expression was detected by Western blotting with anti-p27Kip1 antibody. Expression of actin was used as loading control. Data are representative of 3 experiments. Error bars indicate SEM (n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/8/10.1182_blood-2005-06-2445/4/m_zh80080694160002.jpeg?Expires=1766023358&Signature=XpC5eWk0sIUM2vcl6mvmNtccQFNzdN5Kohun8Ib4E5Ds5nZ0~2-t98hv2lTwbQiZ-VxeCpNPXxCuSzg7imj9JnM1LaGKlYFkW06v~Li30NsYXQ0J-TujxEGQ1uidaNiBpFlVo6vbbmEgQhGDZDciDxR8-VazdpdoUBd68JX6~xz~3D87si~ktO0gzmHbMwaHXQXDLFRXbtkBRqV~l-bJ4DkcRcJPsD68C4x4pGRERjRK3SZYbK2B0mz0wHkFMm~ANTOaaVoCu06hK7Re5wQynharLVkxpSuuab07hwBTIW0vwb7DBJiMPgqCRIXP~WSD-5ExRAahF6oUJLlW~8oBYw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Sema-3A reduces cytokines production. (A) T cells were cultured for the indicated times with anti-CD3 (300 ng/mL) plus anti-CD28 (200 ng/mL) alone or with CM derived from Sema-3A-expressing COS-7 cells as described in Figure 2A. Supernatants were collected, and IL-2, IL-10, IFN-γ, and IL-4 concentrations were assessed by ELISA. (B) T cells were treated as described in panel A for 8 hours. Total RNA was extracted, and IL-2 expression was analyzed by real-time PCR. GAPDH were used as housekeeping gene. *P < .001 versus COS-7/-, ANOVA, n = 3. (C) T cells were cultured for 24 hours with anti-CD3 + anti-CD28 in the presence of CM derived from COS-7/- or COS-7/C1 clones. Recombinant human IL-2, IL-4, and IL-10 (1.0 ng/mL) were added to the culture, and T-cell proliferation was measured by [3H]thymidine incorporation. *P < .05 versus untreated cells, paired t test, n = 3. Error bars indicate SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/8/10.1182_blood-2005-06-2445/4/m_zh80080694160003.jpeg?Expires=1766023358&Signature=C01vEse2mfLpp9a-OHgF3K807433bz1kcwqwilpyM5pdh3QRiMQc9iPxJfhhQpQ9LHw4TO7IkCeSolwvIo~uiesyJax-NZVr-ub-yKRKVQPf2RFVPMOPRKBO1dvp2~o4Gm76zcmxQcGeWHS5eoUmL~jpbWOAxxZfDbbxu83bykytKlS5DhR35VzYUmcgHuspE5l509TSj1xjTXHZkPMd6LJwJ0vLpvqOANG-OaIeMDpKy0N387DBSpZH1JJZoBCraEGq9oS3mYQDL05ITIG4itPJK3h5QLWTToyHBp-tU7gbPN2t5tGZZyIGdeebFIgeC6YYuYJBvHqb8mco4Cpzyw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Down-regulation of Sema-3A expression in tumor cells sensitizes T-cell activation. (A) Caki-1 and H28 cells were transfected with C-siRNA or Sema-3A siRNA (600 nM). Then, immunoprecipitated whole-cell lysates and TSNs were prepared 72 hours after transfection and analyzed as reported in Figure 1. (B) TSNs of transfected H28 cells were collected, and IL-6, TGF-β, and VEGF concentrations were assessed by ELISA. (C-D) T cells were cultured for 24 hours with either anti-CD3 + anti-CD28 alone or with the indicated TSNs (1:2) from Caki-1 cells in the absence or presence of increasing concentrations of Sema3A/Fc or Sema6A/Fc. T-cell proliferation was then assessed by [3H]-thymidine uptake (C). In parallel, supernatants of T cells were collected, and IL-2 and IFN-γ concentrations were assessed by ELISA (D). *P < .001, ANOVA, n = 4. Error bars indicate SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/8/10.1182_blood-2005-06-2445/4/m_zh80080694160005.jpeg?Expires=1766023358&Signature=loI4-nM0vVw0PJHWfKbdvkTUeYGXxxlWHasKp9Du3qls~~WdjkEpr0AD-GwKzYwBZJLQWSWIrHP01Y4YH7XsRzatthmYdNgwXp4FoJdVtNNzwcvz~X2RUP12WgHB22AHTTCm7eBek5I886DDCx8P9U7T~Lm3efeQ3sejgYjtPS32FHEZWcCeWQw-perLyAI4a869B42-t13boj8c-WsZzgh62ujqxlPdPqsHBzdHsHPOFWSorPYGL7dt3mx7IAyAGWZczMSHp6QZRfoJ6vp66M1G9J1eIPTrJz-yz1QWnPh8G-QfyHEGrSdLafH3emshDNPSFrebc~tPY0BCPDZMuA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 2. Sema-3A binds T cells and inhibits T-cell proliferation. (A) Resting T cells were incubated with Sema3A/Fc or hIgG and then analyzed by flow cytometry as described in “Materials and methods.” Mean fluorescence intensity (MFI) derived from 3 independent experiments is shown (left). Alternatively, cells were first incubated with the blocking anti-NP-1 antibody or a control Ab. Then, T cells were treated with Sema3A/Fc or hIgG as described (right). (B) COS-7 cells were stably transfected with a vector that allowed a high (COS-7/C1) and intermediate (COS-7/C2 and COS-7/C3) amount of Sema-3A expression, as shown by Western blot in concentrated serum-free supernatants (CM) (insert). CM (48 hours of conditioning, 1:2 of dilution) derived from COS-7 cells and Sema-3A-expressing COS-7 cells were incubated with T cells for 18 hours in the absence (Control) or in the presence of soluble anti-CD3 (300 ng/mL), either alone or with anti-CD28 (200 ng/mL) or PMA (5 ng/mL). T-cell proliferation was then measured by [3H]thymidine incorporation. *P ≤ .001 versus COS-7/-, ANOVA, n = 3. (C) T cells were treated with CM derived form COS-7/- and COS-7/C1 clones, stained with PI and analyzed by flow cytometry. (D) T cells were treated as described in panel A in the presence of CM derived form COS-7/- and COS-7/C1 clones for the indicated times, and the p21Kip1 protein expression was detected by Western blotting with anti-p27Kip1 antibody. Expression of actin was used as loading control. Data are representative of 3 experiments. Error bars indicate SEM (n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/8/10.1182_blood-2005-06-2445/4/m_zh80080694160002.jpeg?Expires=1766085514&Signature=AGC3u1LjqOFJ-ZsIH3ssn6HR9QCIyWtIGK2pbVo1-6Gz-yYHVk59Tx7-nZ35Bc6mHupXR~35cKH11tUHep7Sjoz~iXQPc54yNdOQppKsE60P4TJI0FdZyvIs28nwzm~c9aslBaZFCJiXRxtX1WG900VQ7ow~HLi3dKeWPdqPmr3V334pnrz7E5vyUar6SepFX5gMt0ZbMhAA~tROv4iQHhTnDgVSyyQYUO0bmikAesTaCmf2x2w~W7yChWr2f7J2dw3uvhegIagP-EXn6mHpRq-skOK~ZtBZYxQv1BxLie51FlLukZIMNC4k5O9yT0qaeay6YQQXmLfR59SKadR7Bw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. Sema-3A reduces cytokines production. (A) T cells were cultured for the indicated times with anti-CD3 (300 ng/mL) plus anti-CD28 (200 ng/mL) alone or with CM derived from Sema-3A-expressing COS-7 cells as described in Figure 2A. Supernatants were collected, and IL-2, IL-10, IFN-γ, and IL-4 concentrations were assessed by ELISA. (B) T cells were treated as described in panel A for 8 hours. Total RNA was extracted, and IL-2 expression was analyzed by real-time PCR. GAPDH were used as housekeeping gene. *P < .001 versus COS-7/-, ANOVA, n = 3. (C) T cells were cultured for 24 hours with anti-CD3 + anti-CD28 in the presence of CM derived from COS-7/- or COS-7/C1 clones. Recombinant human IL-2, IL-4, and IL-10 (1.0 ng/mL) were added to the culture, and T-cell proliferation was measured by [3H]thymidine incorporation. *P < .05 versus untreated cells, paired t test, n = 3. Error bars indicate SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/8/10.1182_blood-2005-06-2445/4/m_zh80080694160003.jpeg?Expires=1766085514&Signature=sjf9RJHXTJXawIqv9IhDUWTbP-V9xoWQe4oZJGT~5lwdcAVwt-3XILxkgpGfLHbyFiSrV-uesb97cnif3~CbZ5EgcoeAC7OR0BYdHvdT-ONvDts~o~mNGtGOyX7h9TgugfPXHuw95OkoaBiPHxTv1-6djCoWeY5tY-dYP3LzucT2e~CHCV1iuYRHPcbuT5LAScMTXBs4TgVCo4N6zwLlWLNdUp8gpvqaHYtgKD-9vpxfmNDlfvgc-NWIRs1lMEq0W5~iJ5RPs0omfMn~LypX~PjycF4xQMN8iCMl6tookBeEhRLPR5a7pRZc3fG7yla5Z-J5I3Eo7AADsLD0erg31Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 5. Down-regulation of Sema-3A expression in tumor cells sensitizes T-cell activation. (A) Caki-1 and H28 cells were transfected with C-siRNA or Sema-3A siRNA (600 nM). Then, immunoprecipitated whole-cell lysates and TSNs were prepared 72 hours after transfection and analyzed as reported in Figure 1. (B) TSNs of transfected H28 cells were collected, and IL-6, TGF-β, and VEGF concentrations were assessed by ELISA. (C-D) T cells were cultured for 24 hours with either anti-CD3 + anti-CD28 alone or with the indicated TSNs (1:2) from Caki-1 cells in the absence or presence of increasing concentrations of Sema3A/Fc or Sema6A/Fc. T-cell proliferation was then assessed by [3H]-thymidine uptake (C). In parallel, supernatants of T cells were collected, and IL-2 and IFN-γ concentrations were assessed by ELISA (D). *P < .001, ANOVA, n = 4. Error bars indicate SEM.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/107/8/10.1182_blood-2005-06-2445/4/m_zh80080694160005.jpeg?Expires=1766085514&Signature=Mzzlhm8gzH6uoaEXCPc8WI~Ub4TFZnCc5Qj1rlrzIOMJoEcFqkiT48QwXBF2hqVxUak0Iui-W0KK8msk2FVek7tKFJwSeKL8xZzK0bLaMLwVuNazQd2gQxx8O259ibwBYxyNZaYHxYfnkeonBVRDoMBuCO95cS4WUmsZiSled468EUcBYTZsV8G333PesL0RAoBVkDib~LFqcBbyuqb63X-wUYTRTYRIFxLL2uoHN3Rvu2kmO43mx837lkLLFZN8o56b240uWaup-DzgYAj4CRly6Zcac7dxPO0PHayL8zRbvBV4jDjoZubkb7gDTRyuYqnvX3bJvAEj5lFAFAQWmA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)