Neonatal alloimmune thrombocytopenia (NAIT) is a fetomaternal incompatibility most commonly induced by maternal anti-HPA-1a, IgG alloantibodies against a polymorphic epitope of the glycoprotein IIb/IIIa complex in approximately 97.5% of white patients. Current guidelines recommend transfusion of immunologically compatible platelets to prevent cerebral hemorrhage, the most severe complication in affected newborns. Such platelet concentrates, however, are often not readily available. In a retrospective analysis in German and Canadian centers, 27 newborns with NAIT were identified who received platelets from random donors. Unexpectedly, 24 of 27 newborns showed an increase above a threshold of 40 × 109 platelets per liter, with moderate (n = 8) or significant (n = 16) platelet count increments (more than 80 × 109/L). We conclude that transfusion of platelet concentrates from random donors is an appropriate strategy in the management of unexpected, severe NAIT predominantly in first pregnancies, pending the availability of compatible platelets.
Introduction
Neonatal alloimmune thrombocytopenia (NAIT) is an immunemediated fetomaternal incompatibility. It occurs after maternal alloimmunization against polymorphic epitopes on fetal platelet glycoproteins (GPs) and diaplacental transfer of maternal IgG alloantibodies to the fetus. Alloantibodies implicated in NAIT are directed against antigens on GP IIb/IIIa, Ib/IX, Ia/IIa, and CD109. The most common antibody is anti-HPA-1a (originally referred to as anti-Zw(a)1 ), which accounts for about 75% of cases.2 A leucine-proline polymorphism of GP IIIa of amino acid 33 is the molecular basis of the HPA-1a/1b polymorphism.3 NAIT has an incidence of 1:1000 to 1:2000 births in white populations and may occur if a pregnant, HPA-1b/1b homozygous woman is immunized with HPA-1a-positive platelets by her fetus.4,5
NAIT is a self-limiting and transient disorder with an excellent prognosis in the absence of cerebral bleeding. Approximately 42% of newborns with NAIT are born to primiparous women.6 Prenatal screening for maternal platelet-specific alloantibodies has not been established,5 and the birth of a first affected child therefore occurs unexpectedly. Because a significant proportion of untreated newborns with NAIT (approximately 7% to 14%6,7 ) are affected by cerebral hemorrhage in the first days of life, a liberal attitude toward platelet transfusion of the severely thrombocytopenic newborn is considered appropriate.8 While intravenous γ-globulin (IVIG) has been shown to be of some benefit in the antenatal management of alloimmune thrombocytopenia,9 high-dose IVIG can only be recommended as a complementary treatment modality in the management of NAIT because of the delayed rise in platelet counts and limited evidence from a small series of cases.10,11 Currently, antigen-negative platelets are considered optimal for the prevention of hemorrhage in newborns with suspected NAIT.8,12 To meet this need, transfusion services have attempted to stock HPA-1a-negative and HPA-5b-negative donor platelet concentrates for emergency use. However, this remains a logistic challenge, given the relatively low frequency (approximately 2.5%) of HPA-1a-negative donors.12 As an alternative to HPA-1a-negative donor platelets, transfusion of maternal platelets is recommended.13 However, these platelets must also be prepared within a short time, and the appropriate facilities are available 24 hours a day only in specialized centers. In addition, maternal platelet concentrates must be processed to remove maternal antibodies, tested for transfusion-relevant infection markers, and irradiated. Therefore, in cases of unexpected NAIT, maternal platelet concentrates are only available after a delay of 12 to 48 hours.
While awaiting the arrival of compatible platelets, newborn babies with alloimmune thrombocytopenia due to maternal anti-HPA-1a have been transfused with random donor platelets, and in some cases unexpected high platelet increments have been observed.14-17 To study more systematically the effect of HPA-1a-incompatible platelet transfusions in severe NAIT, we initiated a review of newborns with severe NAIT who received random donor platelet concentrates.
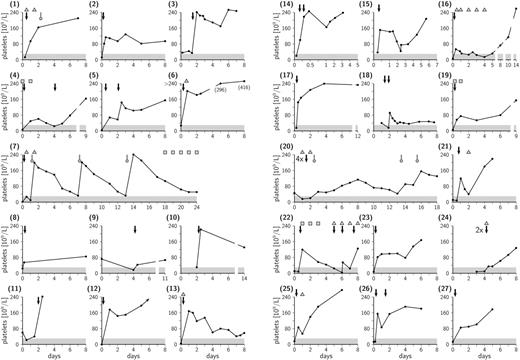
Platelet count and therapy in case nos. 1 to 27. HPA-1a-positive platelet transfusions are indicated by black arrows and HPA-1a-negative transfusions by gray arrows with round heads. IVIG infusions are indicated by triangles and single doses of corticosteroids by squares. Newborns with platelet counts below 30 × 109/L (area shaded in gray) are considered to be at enhanced risk for major hemorrhage.
Platelet count and therapy in case nos. 1 to 27. HPA-1a-positive platelet transfusions are indicated by black arrows and HPA-1a-negative transfusions by gray arrows with round heads. IVIG infusions are indicated by triangles and single doses of corticosteroids by squares. Newborns with platelet counts below 30 × 109/L (area shaded in gray) are considered to be at enhanced risk for major hemorrhage.
Study design
In this retrospective analysis we enrolled 1 Canadian (Hamilton) and 6 German (Berlin, Bonn, Düsseldorf, Giessen, Greifswald, Rostock) university hospitals and 1 German Red Cross transfusion laboratory (Dresden). Each center identified platelet transfusions in NAIT patients who fulfilled the following criteria: (1) laboratory-confirmed HPA-1a antibodies in maternal-blood samples, (2) transfusion of platelet concentrate(s) from random donors, and (3) a period of observation of 4 days or longer with documented platelet counts. Platelet transfusions and other therapeutic interventions aimed at preventing bleeding complications, platelet counts, and response to platelet concentrate transfusions were retrieved from each patient's file. Platelet antibodies were detected using GP-specific assays.18
Results and discussion
Twenty-seven neonates (11 female, 16 male) born to mothers with serologically confirmed HPA-1a antibodies were identified (Figure 1) who received at least 1 HPA-1a-positive (patient nos. 1-3, 5, 8-11, and 13) or random platelet concentrate. Maternal antibody status was known in 5 of 27 cases. The median gestational age was 39 weeks; 25th quartile, 37.5 weeks; 75th quartile, 39.5 weeks. Thirteen of 26 newborns were born to primiparous women. In all but 3 patients (nos. 7, 20, and 24) platelet counts increased above 40 × 109/L following 1 or 2 random platelet transfusions, a value above the threshold of 30 × 109/L considered relevant in the prevention of cerebral hemorrhage.8 After the first 1 or 2 transfusions, significant increments (more than 80 × 109/L) were observed in 16 of 27 patients (nos. 1-3, 5, 6, 10-15, 17, 21, 23, 25, and 26), and less pronounced but still sufficient increments were seen in patients 4, 8, 9, 18, and 19. Even patient 16, the second of 2 siblings with severe NAIT, who was born with a platelet count of 6 × 109/L and who showed a minor increment, still did not require further transfusion. Response to 4 random platelet transfusions was highly variable in patient 22. There were 3 exceptions in this series of patients: in patients 7, 20, and 24 random donor platelets were clearly without effect, and further HPA-1a-negative platelet transfusions were required in patients 7 and 20. Ten patients also received IVIG (nos. 1, 6, 7, 13, 16, 20-22, 24, and 25), and 4 patients were treated with corticosteroids (nos. 4, 7, 19, and 22). In none of the patients were adverse effects related to random donor platelet transfusions (eg, disseminated intravascular coagulation [DIC] or increased hemorrhage) observed. Cerebral hemorrhage occurred prenatally in patients 1 and 6, and in patient 5 hydrocephalus of unknown etiology was diagnosed at birth, underscoring the severity of NAIT.
The cause for the relative effectiveness of immunologically incompatible platelet transfusion in NAIT is not entirely clear. Potentially a sufficiently large dose of antigen-positive platelets might adsorb circulating alloantibodies and thus enhance recovery of megakaryocytes and thrombocytopoiesis. This could explain the observation of a delayed rise in platelet counts in some patients. From an immunologic perspective, a newborn who has been sensitized by alloantibodies as result of passive transplacental transfer may react more favorably when transfused with antigen-positive platelets than an actively immunized subject. The likely explanation is that the incompatible transfusion in the situation of passive immunization will not enhance the antibody titer.
In our study, we found that in 24 of 27 newborns with unexpected severe NAIT, transfusion of a random platelet concentrate led to an increase in platelet count sufficient for the prevention of spontaneous cerebral hemorrhage. This and the fact that transfused babies experienced no serious adverse effects strongly indicate that immediate transfusion of a random platelet concentrate in severe, unexpected NAIT may be associated with fewer risks than waiting for several hours or even days for a HPA-compatible platelet concentrate. Our study clearly demonstrates that a positive response to antigen-incompatible platelets does not exclude NAIT and confirms observations by Bussel et al.19 Although the cases described in this paper may not include all presentations of NAIT, they lend credence to the fact that transfusion of antigen-positive platelets is a definite treatment option in NAIT following maternal HPA-1a alloimmunization.
In conclusion, we recommend the use of random platelet concentrates in newborns suspected to have NAIT with platelet counts below 30 × 109/L while waiting for matched (HPA-1a-negative), HPA-5b-negative) platelets if these are not immediately available. One might speculate that transfusion of antigen-positive platelets will shorten the thrombocytopenic period if adsorption of alloantibodies is the major mechanism. A randomized controlled trial to support the efficacy and safety of this strategy is awaited, and the benefits of concomitant administration of IVIG remain to be demonstrated.
Prepublished online as Blood First Edition Paper, January 10, 2006; DOI 10.1182/blood-2005-06-2235.
Supported by the German Federal Ministry of Education and Research (CAN04/006; NLB3 program ref 01-ZZ0403).
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The largest group of patients was contributed by H. Kroll, MD (now located at German Red Cross Blood Transfusion Service, Dessau, Germany).