This study presents evidence that human platelets bind lipopolysaccharide (LPS) from enterohemorrhagic Escherichia coli (EHEC) through a complex of toll-like receptor 4 (TLR4) and CD62, leading to their activation. TLR4 colocalized with CD62 on the platelet membrane, and the TLR4 specificity of LPS binding to platelets was confirmed using C57BL/10ScN mice lacking Tlr4. Only platelets from TLR4 wild-type mice bound O157LPS in vitro. After in vivo injection, O157LPS bound to platelets from wild-type mice, which had lower platelet counts than did mice lacking TLR4. Mouse experiments confirmed that O157LPS binding to TLR4 is the primary event leading to platelet activation, as shown by CD40L expression, and that CD62 further contributes to this process. Activation of human platelets by EHEC-LPS was demonstrated by expression of the activated GPIIb/IIIa receptor, CD40L, and fibrinogen binding. In perfusion experiments, platelet activation on endothelial cells was TLR4 and CD62 dependent. O157LPS was detected on platelets from 12 of 14 children with EHEC-associated hemolytic uremic syndrome (HUS) and on platelets from 2 children before the development of HUS but not on platelets of EHEC-infected children in whom HUS did not develop (n = 3). These data suggest that O157LPS on platelets might contribute to platelet consumption in HUS. (Blood. 2006;108:167-176)
Introduction
Hemolytic uremic syndrome (HUS) classically occurs after diarrhea caused by enterohemorrhagic Escherichia coli (EHEC) infection; O157:H7 is the most common serotype. HUS is characterized by nonimmune microangiopathic hemolytic anemia, thrombocytopenia, and acute renal failure.1 Before the onset of clinical HUS, thrombin production is increased, and fibrinolysis is inihibited.2 During HUS, thrombocytopenia is accompanied by an increase in fibrin degradation products3 without excessive consumption of fibrinogen. An imbalance between prothrombotic and fibrinolytic plasma components presumably contributes to a prothrombotic, hypofibrinolytic state.4,5 Platelet consumption in HUS may be induced by this imbalance, endothelial cell injury, or direct platelet activation by bacterial or host factors.6
Several groups have reported platelet activation in HUS, as evidenced by elevated levels of platelet factor 4, β-thromboglobulin, soluble P-selectin, and platelet-derived microvesicles.7-9 Direct activation by bacterial virulence or host factors might contribute to platelet activation and consumption. We have shown that Shiga toxin (Stx) induces platelet activation.10 Other Escherichia coli O157:H7 virulence factors, such as LPS, have not been studied. This is particularly interesting because endotoxemia has been reported in patients with Shigella dysenteriae-associated HUS.11
LPS is an abundant outer membrane component of Gramnegative bacteria. Its potent biologic activity is related to its ability to trigger the innate immune system to initiate an inflammatory response.12 LPS is found in the circulation on bacterial outer membranes or in aggregates. LPS-binding protein (LBP) binds monomers of LPS, after which a complex with CD14 is formed.12 CD14 is a glycosylphosphatidylinositol-anchored cell surface molecule expressed on neutrophils and monocytes. It forms a complex with toll-like receptor 4 (TLR4) and MD2, which enables intracellular signaling.12 CD14- cells do not respond to LPS but might respond to this stimulus in the presence of soluble CD14 (sCD14) in the circulation.13
The aim of this study was to determine whether EHEC LPS interacts with human platelets and whether this interaction triggers platelet activation. In particular, the study was designed to examine the mechanism by which EHEC LPS binds to platelets, to investigate LPS-induced platelet activation, and to define whether this activity is specific for the E coli O157 serotype. In addition, the presence of LPS was examined on platelets from patients infected with EHEC.
Patients, materials, and methods
Blood collection and platelet preparation
Blood from 36 healthy adult donors, 19 patients, and 5 pediatric controls was collected by venipuncture into Vacutainer tubes (Becton Dickinson, Plymouth, United Kingdom; Becton Dickinson, Franklin Lakes, NJ) containing 0.5 mL of 0.129 M sodium citrate. Samples were centrifuged at 200g at room temperature for 20 minutes, and platelet-rich-plasma (PRP) was removed. PRP was used in flow cytometry, immunofluorescence microscopy, or epifluorescence video microscopy experiments or was further centrifuged at 2000g for 10 minutes to obtain platelet-poor plasma and washed platelets (WPs).10 WPs were resuspended in sterile LPS-free phosphate-buffered saline (PBS; pH 7.4; Medicago, Uppsala, Sweden) for electron microscopy or epifluorescence video microscopy experiments and in healthy donor plasma for flow cytometry experiments.
Platelets were used at a concentration of 1 × 108/mL, adjusting the concentration with platelet-poor donor plasma (for PRP) or with PBS or platelet-poor plasma (for WPs). Platelets were identified by labeling with mouse anti-human CD41-PE antibody (1:10; Immunotech, Marseilles, France) in flow cytometry or fluorescence microscopy experiments.
Centrifugation of whole blood to PRP and washing of platelets before and after resuspension in plasma did not lead to activation, as assessed by the expression of P-selectin (mouse anti-human CD62 1:10; Immunotech), fibrinogen binding (chicken anti-human fibrinogen-FITC 1:700; Diapensia HB, Linköping, Sweden), or thrombospondin (rabbit anti-human thrombospondin 20 μg/mL; Calbiochem, La Jolla, CA). To induce the expression of platelet receptors, WPs were stimulated with thrombin (1 U/mL; Sigma, St Louis, MO) for 2 minutes at 25°C, washed, and resuspended in the healthy donor plasma or PBS.
Subjects
Blood samples were available from 7 boys and 7 girls, aged 1 to 13 years (median, 4 years) treated for EHEC infection at the Department of Pediatrics, Lund University Hospital. All had diarrhea. Samples were taken within 2 days of admission, while 13 of 14 children still had diarrhea and all but 1 had clinical signs of HUS. HUS was defined as hemolytic anemia (hemoglobin level less than 100 g/L), thrombocytopenia (platelet count less than 150 × 109/L), and azotemia. EHEC infection was detected as previously described.26,27 One patient with hemorrhagic colitis did not develop complete HUS, but platelet counts decreased from 371 to 195 × 109/L; mild anemia developed (hemoglobin level, 101 g/L), lactic dehydrogenase increased (from 11 to 16 μkat/L), and creatinine increased from 35 to 64 μM/L. Three months to 1 year after recovery, PRP was obtained from 5 of the children with E coli O157 infection.
Platelet samples were also available from 2 children, a 4-year-old girl and a 2-year-old boy, with E coli-associated HUS treated at the Children's Hospital and Regional Medical Center (CHRMC) in Seattle. Samples were taken during the acute phase of disease while both children still had diarrhea. In the girl, the sample was taken 1 day before HUS developed, and in the boy samples were available 3 days before HUS and on the day HUS developed. In addition, platelets were obtained from 3 girls—ages 3, 7, and 8 years—treated at CHRMC for uncomplicated EHEC infection (diarrhea without HUS).
Blood was available from 5 pediatric controls, 2 boys and 3 girls ages 5 to 9 years (median, 6 years). These children had neither diarrhea nor HUS and were seen (Section of Nephrology, Department of Pediatrics, Lund University Hospital) for follow-up of other conditions. Samples from healthy donors, patients, and pediatric controls were obtained with the informed consent of the donors and the parents. The study was approved by the Ethics Committee of Lund University and the Institutional Review Board of CHRMC.
Immunofluorescence microscopy
PRP from healthy donors, patients, and pediatric controls at 1 × 108/mL was centrifuged onto glass slides (Cytospin, Shandon, Pittsburgh, PA), fixed, blocked, and washed.10 Patient slides were incubated separately with mouse anti-O157LPS (20 μg/mL),14 mouse anti-Stx1 (15 μg/mL; Toxin Technology, Sarasota, FL), mouse anti-Stx2 (200 ng/mL; gift from T. G. Obrig, Department of Nephrology, University of Virginia, Charlottesville, VA), or mouse IgG1 (20 μg/mL; Dako, Glostrup, Denmark) as the negative control for all 3 antibodies, mouse anti-human CD62-FITC (10 μg/mL; Dako) or mouse IgG1-FITC (10 μg/mL; Dako) as its isotype control, and chicken anti-human fibrinogen-FITC (1: 700)10,15 or chicken anti-human insulin-FITC (1:700, Diapensia) as its negative control. Similarly, PRP from healthy donors was incubated with O157LPS14 (final concentration, 1 μg/mL) or PBS for 45 minutes at 37°C, washed, and incubated with anti-O157LPS. PRP from healthy donors was tested for the presence of CD14 on the surface (mouse anti-human CD14, 1:10; Dako). Slides were incubated with the primary antibody for 1 hour at 37°C. After 3 washes with PBS, the slides were incubated for 1 hour at 37°C with goat F(ab′)2 anti-mouse IgG-FITC (1:20; Dako) as the secondary antibody if the primary antibody was not conjugated. Slides were washed 3 times, dried, covered with fluorescent mounting medium (Dako), and examined under an Axiostar plus fluorescence microscope equipped with a 40 ×/0.70 objective lens and an Axiocam MRc5 camera (Carl Zeiss, Göttingen, Germany). AxioVision AC software version 4.4 (Carl Zeiss) was used for image processing.
Flow cytometry
LPS binding. LPS binding to human platelets was assayed using platelets in PRP or WPs. PRP was incubated with purified LPS from E coli O103, O111, O121,16,17 O157 (100 ng or 1 μg/mL; final concentrations determined by the limulus amebocyte lysate assay; Coatex AB, Gothenburg, Sweden) or non-EHEC-LPS O111:B4 (Sigma) for 45 minutes at 37°C, gently mixed every 10 minutes, washed in PBS, and fixed in paraformaldehyde (Sigma).10 For saturation experiments, O157LPS was added to PRP to obtain a final concentration between 100 pg and 200 μg/mL. The kinetics of O157LPS binding to platelets was investigated by incubating 1 μg/mL O157LPS with PRP for 5 minutes to 2 hours. In other experiments, WPs were thrombin stimulated or unstimulated, washed, and resuspended in the donor plasma containing O157LPS (1 μg/mL) for 45 minutes and were fixed. In these experiments, plasma was added as a source of LPS-binding protein.18
After incubation with LPS, platelets were incubated with mouse anti-O157LPS or polyclonal OK rabbit antiserum E coli R1 to detect non-O157 serotypes (1:200; Statens Serum Institute, Copenhagen, Denmark) for 1 hour at 37°C. Cells were then washed and incubated with goat F(ab′)2 anti-mouse IgG-FITC (1:20) or swine anti-rabbit IgG-FITC (1:40; Dako) for 20 minutes at room temperature. Analyses were performed with a FACSCalibur instrument with CellQuest software (Becton Dickinson Immunocytometry Systems, San Jose, CA). In all experiments, binding was calculated after subtracting background fluorescence of the irrelevant antibody.
Specificity of the anti-O157LPS antibody was tested by preincubating the antibody with O157LPS at an antibody/LPS ratio of 20:1 for 1 hour. The combined antibody/LPS was then incubated with PRP for 45 minutes and compared with PRP incubated with LPS alone. Platelets were then washed, fixed, and incubated with monovalent rabbit antisera O157-56 (1:100; Statens Serum Institute) followed by swine anti-rabbit IgG-FITC.
CD14 and TLR4 expression. CD14 expression on platelets was examined by incubation of PRP, alternatively washed and thrombinstimulated or unstimulated fixed platelets, with anti-CD14, as described for immunofluorescence experiments. Similarly, PRP was tested for the presence of CD14 after preincubation with LPS 1 μg/mL for 45 minutes. Detection of TLR4 on platelets was performed by incubation with mouse anti-human TLR4 (20 μg/mL; eBioscience, San Diego, CA) or rabbit anti-human TLR4 (20 μg/mL; eBioscience). Mouse IgG2a (Dako) or rabbit IgG (Dako) was used as irrelevant antibodies. After washing, platelets were incubated with goat F(ab′)2 anti-mouse IgG-FITC or swine anti-rabbit IgG-FITC.
CD62 (P-selectin) expression. CD62 expression was detected by incubation of washed thrombin-stimulated or unstimulated platelets with mouse anti-human CD62 followed by incubation with goat F(ab')2 anti-mouse IgG-FITC.
Specificity of LPS binding to TLR4 or CD62. The specificity of LPS binding to TLR4 or CD62 was assessed by preincubating washed thrombinstimulated platelets with rabbit anti-human TLR4, rabbit anti-human CD62 (20 μg/mL; BD Biosciences, San Jose, CA), or rabbit IgG as an irrelevant antibody for 1 hour at 37°C. Platelets were then washed and resuspended in the donor plasma containing O157LPS (1 μg/mL) for 45 minutes. Platelets were further washed and incubated with mouse anti-O157LPS followed by goat F(ab')2 anti-mouse IgG-FITC. Similarly, the polyclonal antibodies were substituted by mouse anti-human TLR4 or mouse anti-human CD62 (20 μg/mL; BD Biosciences). Cross-competition experiments were carried out in which platelets were incubated with O157LPS and rabbit or mouse anti-human CD62 simultaneously.
Markers of platelet activation. The effect of LPS on platelet activation was studied by the expression of the activated GPIIb/IIIa receptor (PAC-1 antibody), CD40L, and fibrinogen binding. PRP was first preincubated with or without LPS followed by incubation with mouse anti-human PAC-1 IgM (15 μg/mL; BD Biosciences), rabbit anti-human CD40L (1:20; Santa Cruz Biotechnology, Santa Cruz, CA), or chicken anti-human fibrinogen-FITC. Secondary antibodies were rat anti-mouse IgM-FITC (1:20; eBioscience) or swine anti-rabbit IgG-FITC. Thrombin (1 U/mL)-stimulated WPs were used as the positive control.
Electron microscopy
Grids coated with washed thrombin-stimulated platelets were prepared for immunostaining19 by simultaneous overnight incubation at 4°C with mouse anti-human TLR4 (1:20) and rabbit anti-human CD62 (1:20) and with each antibody separately. Grids were separately or simultaneously incubated with control mouse IgG1 and rabbit IgG. As a control for receptor colocalization, separate grids were incubated simultaneously with mouse anti-human CD63 (1:20; Santa Cruz Biotechnology) and rabbit anti-human CD40L (1:20). After washing, the grids were simultaneously incubated with gold-conjugated secondary antibodies (1:20; goat anti-mouse-gold 6 nm and goat anti-rabbit-gold 10 nm [BB International, Cardiff, United Kingdom]) for 1 hour. Specimens were observed with a JEM 1230 electron microscope (JEOL, Tokyo, Japan) operated at 80 kV accelerating voltage, and images were recorded with a Multiscan 791 CCD camera (Gatan, Pleasanton, CA). Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) was used for image processing. Gold particles (6 and 10 nm) on the cell membranes were not considered associated if they were separated by more than 30 nm.20
Mice
C3H/HeN, C3H/HeJ, C57BL/10ScSn, and C57BL/10ScN female and male mice between 6 and 12 weeks of age were used. C3H/HeN and C57BL/10ScSn are wild-type strains. C3H/HeJ and C57BL/10ScN are LPS-hyporesponsive or unresponsive (respectively) compared with their wild-type strains. The C3H/HeJ mouse has a spontaneous point mutation of the TLR4 protein leading to defective LPS signaling.21 C57BL/10ScN mice lack the entire Tlr4 gene.21
All mice were bred at the Department of Laboratory Medicine, Lund University. Mice were anesthetized by intraperitoneal midazolam 5 mg/kg, fentanyl citrate 0.315 mg/kg, and fluanisone 10 mg/kg. In the first category of experiments, blood was taken from all 4 strains of mice to study O157LPS binding and platelet activation. CD14 expression was examined by incubating washed thrombin-stimulated (0.2 U/mL) platelets with rat anti-mouse CD14-FITC (1:50; BD Biosciences). Washed thrombinstimulated platelets were also tested for the presence of CD14 after incubation with O157LPS diluted in autologous plasma. The importance of CD62 for LPS binding was tested in thrombin-activated mouse platelets by preincubation of the washed platelets with rat anti-mouse CD62 (10 and 50 μg/mL; BD Biosciences) before LPS was added. Detection of platelet activation was performed in resting platelets with hamster anti-mouse CD40L (10 μg/mL; BD Biosciences), followed by mouse anti-hamster-FITC (1:80; BD Biosciences). To block nonspecific binding of immunoglobulins to FcγRIII and FcγRII receptors, platelets were incubated with rat anti-mouse CD16/CD32 (15 μg/mL; BD Biosciences) before antibodies were added. In the second group of experiments, all 4 mouse strains were given intraperitoneal injections of O157LPS (30 μg/kg) 0.75 μg/mouse diluted in sterile PBS or PBS alone. After 4 hours, mice were anesthetized and blood was taken. These studies were approved by the Animal Ethics Committee of Lund University.
Epifluorescence video microscopy
Platelet binding to O157LPS (510 ng/mL) bound to human microvascular endothelial cell-1 (HMEC-1 [Centers for Disease Control and Prevention, Atlanta, GA] cultured in M199 medium [Invitrogen, Carlsbad, CA] containing 50% human serum) was analyzed in PRP and WPs under various flow conditions using a modification of a parallel-plate flow chamber, as described.22,23 Platelet binding was visualized in real time with an Eclipse TE200 inverted microscope equipped with a 40 ×/0.75 objective lens (Nikon, Tokyo, Japan) and an ORCA-ER digital camera (Hamamatsu, Hamamatsu City, Japan). Simple PCI 4.0 software (Compix, Sewickley, PA) was used for image processing. Inhibition experiments were performed by incubation of PRP with mouse anti-human TLR4 or CD62 (both at 50 μg/mL) for 20 minutes at room temperature before perfusion.
Statistical analysis
Differences between LPS-treated and untreated human platelets and between wild-type and mutated mouse platelets were assessed by the Mann-Whitney U test. A P value of .05 or lower was considered significant. Statistical analyses were performed using SPSS version 11 (SPSS, Chicago,IL). The association of 2 different receptors on the platelet membrane was assessed by comparison of the 2 proportions; a normally distributed test quantity was calculated.24
Results
LPS binds to platelets from healthy donors
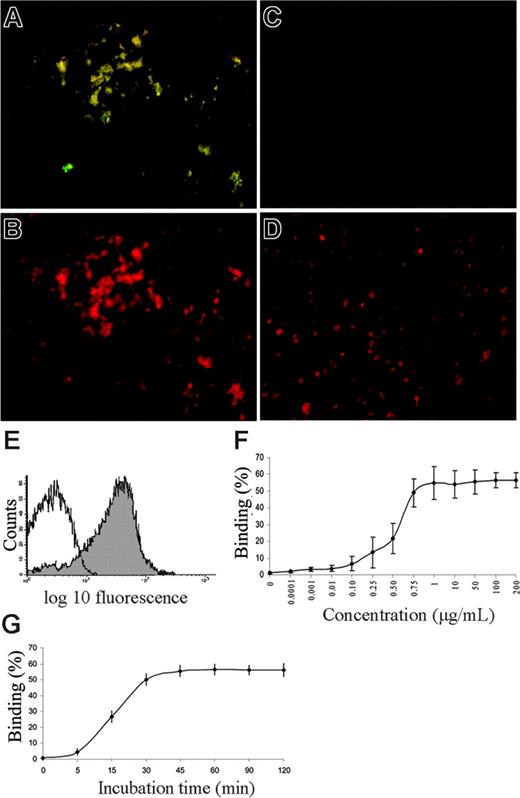
LPS binding to healthy platelets was visible by immunofluorescence microscopy at 1 μg/mL (Figure 1A-B). O157LPS bound to PRP (median, 62%; range, 23%-93%; 23 experiments; Figure 1E; Table 1) as determined by flow cytometry. Binding of O157LPS to washed platelets (in donor plasma) increased after thrombin stimulation (median, 91%; range, 84%-96%; 7 experiments) compared with unstimulated platelets (median, 63%; range, 61%-70%; 7 experiments; P < .002). The higher concentration of O157LPS (1 μg/mL) exhibited more binding to platelets than the other LPS serotypes (Table 1). Differences between untreated platelets in the various groups could be attributed to different anti-LPS antibodies (anti-O157LPS or OK rabbit antiserum E coli R1) and donor platelet variations. O157LPS binding was dose dependent and saturable at 1 μg/mL (Figure 1F). Maximal binding of O157LPS to platelets was achieved after 30 minutes (Figure 1G) and did not decrease over the subsequent 2 hours. Preincubation of O157LPS with O157LPS antibody reduced binding of LPS to platelets (median 79% reduction, from 93% to 19% binding; 4 experiments, P < .02).
LPS binding to normal platelets in vitro, saturability, and kinetics. (A) PRP from a healthy donor incubated with O157LPS at 1 μg/mL and stained with anti-O157LPS. Platelet aggregates are visible by immunofluorescence. (B) Same as for panel A, labeled with CD41-PE to identify platelets. (C) Untreated platelets (PRP) from a healthy donor labeled with anti-O157LPS antibody. (D) Same as for panel C labeled, with CD41-PE. No aggregates are visible. (E) Results of a representative experiment of PRP from a healthy donor incubated with LPS (1 μg/mL) showing O157LPS binding to 75% of the platelet population (filled) compared with the control antibody (open). (F) Saturability was obtained at an O157LPS concentration of approximately 1 μg/mL. Vertical lines denote the standard deviation. (G) Kinetics of O157LPS binding to platelets showing that maximal binding was achieved after 30 minutes' incubation and did not decrease within 2 hours.
LPS binding to normal platelets in vitro, saturability, and kinetics. (A) PRP from a healthy donor incubated with O157LPS at 1 μg/mL and stained with anti-O157LPS. Platelet aggregates are visible by immunofluorescence. (B) Same as for panel A, labeled with CD41-PE to identify platelets. (C) Untreated platelets (PRP) from a healthy donor labeled with anti-O157LPS antibody. (D) Same as for panel C labeled, with CD41-PE. No aggregates are visible. (E) Results of a representative experiment of PRP from a healthy donor incubated with LPS (1 μg/mL) showing O157LPS binding to 75% of the platelet population (filled) compared with the control antibody (open). (F) Saturability was obtained at an O157LPS concentration of approximately 1 μg/mL. Vertical lines denote the standard deviation. (G) Kinetics of O157LPS binding to platelets showing that maximal binding was achieved after 30 minutes' incubation and did not decrease within 2 hours.
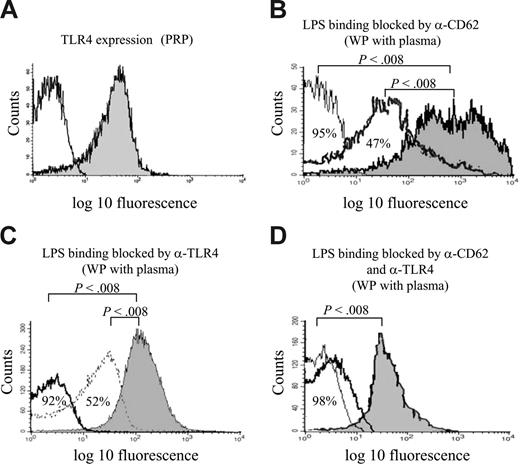
LPS binding to platelets by TLR4 and CD62. (A) PRP incubated with mouse anti-human TLR4 (filled) or isotype control (open) showing binding of the TLR4 antibody to 51% of the platelet population. (B) Preincubation of washed thrombin-stimulated platelets with anti-CD62 at saturating (30 μg/mL, thin line) and subsaturating (10 μg/mL, bold line) concentrations reduced LPS binding by 95% and 47%, respectively, compared with platelets that were not preincubated (filled). (C) Preincubation of washed thrombin-stimulated platelets with anti-TLR4 at saturating (30 μg/mL, bold line) and subsaturating (7.5 μg/mL, thin line) concentrations reduced LPS binding by 92% and 52%, respectively, compared with platelets that were not preincubated (filled). (D) Preincubation with anti-TLR4 and anti-CD62, simultaneously, reduced LPS binding at saturating (thin) and subsaturating (bold) concentrations (98% and 96% reduction, respectively). In all experiments in which washed platelets were used (B-D), LPS was diluted in donor plasma.
LPS binding to platelets by TLR4 and CD62. (A) PRP incubated with mouse anti-human TLR4 (filled) or isotype control (open) showing binding of the TLR4 antibody to 51% of the platelet population. (B) Preincubation of washed thrombin-stimulated platelets with anti-CD62 at saturating (30 μg/mL, thin line) and subsaturating (10 μg/mL, bold line) concentrations reduced LPS binding by 95% and 47%, respectively, compared with platelets that were not preincubated (filled). (C) Preincubation of washed thrombin-stimulated platelets with anti-TLR4 at saturating (30 μg/mL, bold line) and subsaturating (7.5 μg/mL, thin line) concentrations reduced LPS binding by 92% and 52%, respectively, compared with platelets that were not preincubated (filled). (D) Preincubation with anti-TLR4 and anti-CD62, simultaneously, reduced LPS binding at saturating (thin) and subsaturating (bold) concentrations (98% and 96% reduction, respectively). In all experiments in which washed platelets were used (B-D), LPS was diluted in donor plasma.
Mechanism of O157LPS binding
Expression of TLR4. Platelets express TLR4, as demonstrated in PRP by binding of the anti-TLR4 antibody (median, 43%; range, 24%-60%, 19 experiments, Figure 2A). Binding of the anti-TLR4 antibody increased when WPs were thrombin stimulated (median, 79%; range, 64%-85%; 19 experiments vs median for unstimulated WPs of 30%; range, 12%-48%; 12 experiments; P < .001). Platelets (PRP and washed thrombin-stimulated platelets) did not bind antibody to CD14 by flow cytometry (median, 1.3% binding; range, 0%-2%; 10 experiments) or by immunofluorescence microscopy (5 experiments). However, PRP preincubated with LPS 1 μg/mL bound CD14 (median, 54%; range, 46%-59%; 5 experiments), as detected by flow cytometry. Preincubation of PRP with anti-CD14 did not abrogate LPS binding (median, 51%; range, 47%-67%; 5 experiments), indicating that the CD14 on platelets after incubation with LPS was not membrane-bound but, rather, soluble CD14.
CD62 and TLR4 colocalize on the platelet membrane. (A) Thrombin-stimulated platelets were simultaneously incubated with mouse anti-human TLR4 and rabbit anti-human CD62 showing colocalization on the platelet membrane. (Inset) Complex composed of 2 gold-labeled anti-CD62 antibodies (10 nm) and 2 anti-TLR4 antibodies (6 nm). (B) Colocalization of TLR4 and CD62 demonstrated on another platelet. (C) Washed thrombin-stimulated platelets were simultaneously incubated with the control antibodies mouse IgG1 and rabbit IgG. Nonspecific binding was noted, and colocalization of the antibodies was not observed.
CD62 and TLR4 colocalize on the platelet membrane. (A) Thrombin-stimulated platelets were simultaneously incubated with mouse anti-human TLR4 and rabbit anti-human CD62 showing colocalization on the platelet membrane. (Inset) Complex composed of 2 gold-labeled anti-CD62 antibodies (10 nm) and 2 anti-TLR4 antibodies (6 nm). (B) Colocalization of TLR4 and CD62 demonstrated on another platelet. (C) Washed thrombin-stimulated platelets were simultaneously incubated with the control antibodies mouse IgG1 and rabbit IgG. Nonspecific binding was noted, and colocalization of the antibodies was not observed.
LPS binding to CD62. LPS has been proposed to recognize CD62.25 CD62 expression on the platelet surface was increased by thrombin stimulation (median, 95%; range, 92%-99%; 12 experiments) in contrast to WPs that were not thrombin stimulated (median, 0.05%; range, 0%-0.09%; P < .001; 12 experiments). The involvement of CD62 in LPS binding to platelets was investigated, as shown in Figure 2B and Table 2. Results show that the polyclonal, but not the monoclonal, anti-CD62 antibody blocked LPS binding (probably because the polyclonal antibody blocked the LPS binding epitope) and that LPS could block the binding of anti-CD62 antibodies, suggesting that LPS occupied the antibodies' binding site.
LPS binding to TLR4. In addition to binding through CD62, LPS binds to platelets through TLR4. Preincubating washed thrombin-stimulated platelets with rabbit anti-TLR4 before adding O157LPS diluted in donor plasma reduced O157LPS binding (Figure 2C). Simultaneous preincubation of platelets with subsaturating concentrations of polyclonal anti-TLR4 and anti-CD62 abrogated LPS binding (Figure 2D), suggesting that CD62 and TLR4 may have a functional association. The binding of each antibody was not altered (data not shown). The control antibody did not block LPS binding (data not shown). Furthermore, preincubating platelets with O157LPS led to decreased binding of anti-TLR4 from a median of 76% (range, 65%-84%) to 4% (range, 3%-6%; P < .002; 7 experiments).
Colocalization of CD62 and TLR4 on the platelet membrane. Abrogation of LPS binding by anti-TLR4 and anti-CD62 suggested that these receptors might be in complex on the platelet membrane. Surface expression of TLR4 and CD62 receptors was visualized on membranes of thrombin-stimulated platelets with the use of electron microscopy, and colocalization was detected (Figure 3A-B). The percentage of colocalization was determined by the frequency of 6-nm gold particles associated with 10-nm gold particles on 50 cell profiles. Of all counted TLR4 particles, 47% were associated with CD62 on platelet membranes. CD62 and TLR4 labeling did not colocalize intracellularly. In comparison, only 4% of CD63 particles associated with CD40L (P < .001). Control antibodies bound nonspecifically and did not colocalize (Figure 3C). Results indicate a complex formation between TLR4 and CD62 on the platelet surface.
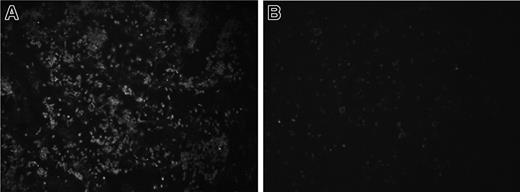
TLR4 is required for LPS binding and reduction of platelet counts. The importance of TLR4 for LPS binding was examined by comparing platelets from C3H/HeJ (with a mutated TLR4) and C57BL/10ScN (lacking TLR4) mice with platelets from wild-type mice. Platelets from C3H/HeN, C3H/HeJ, and C57BL/10ScSn mice bound similar amounts of LPS whereas platelets from C57BL/10ScN mice did not bind LPS (Table 3), indicating that the TLR4 receptor is required for binding. These results were further confirmed when all mice were given intraperitoneal injections of O157LPS. Platelets from C3H/HeN, C3H/HeJ, and C57BL/10ScSn mice bound LPS, but platelets from the C57BL/10ScN strain did not (Figure 4A-B). Wild-type C3H/HeN and C57BL/10ScSn mice given injections of O157LPS developed ruffled fur, lethargy, stillness, and hunched back, whereas C3H/HeJ and C57BL/10ScN mice remained asymptomatic. Platelet counts of C3H/HeN and C57BL/10ScSn mice were reduced compared with those of the C3H/HeJ and C57BL/10ScN strains, as shown in Table 4. These results show that LPS binding to platelets depends on TLR4, and that wild-type mice, capable of binding and responding to LPS, had lower platelet counts than did Tlr4-mutated mice.
TLR4 is required for LPS binding to mouse platelets. C57BL/10ScSn (n = 9) and C57BL/10ScN (n = 8) mice were given intraperitoneal injections of O157LPS, and platelets were taken after 4 hours and fixed on glass slides, as described for human PRP. PRP from a C57BL/10ScSn wild-type mouse (A) incubated with anti-O157LPS antibody showed aggregates of platelets labeled with O157LPS. No labeling of the O157LPS antibody was detected on platelets from a C57BL/10ScN mouse (B).
TLR4 is required for LPS binding to mouse platelets. C57BL/10ScSn (n = 9) and C57BL/10ScN (n = 8) mice were given intraperitoneal injections of O157LPS, and platelets were taken after 4 hours and fixed on glass slides, as described for human PRP. PRP from a C57BL/10ScSn wild-type mouse (A) incubated with anti-O157LPS antibody showed aggregates of platelets labeled with O157LPS. No labeling of the O157LPS antibody was detected on platelets from a C57BL/10ScN mouse (B).
CD62 contributes to LPS binding in activated platelets. Unstimulated platelets from C57BL/10ScN mice did not exhibit LPS binding, but thrombin stimulation led to significant binding, suggesting that CD62 could be involved (Table 3; P < .001). Preincubation of platelets with anti-CD62 reduced LPS binding, as shown in Table 3.
LPS induces platelet activation
Incubation of PRP with different serotypes of LPS led to activation, as demonstrated by expression of the GPIIb/IIIa receptor, CD40L, and fibrinogen binding (Table 5).
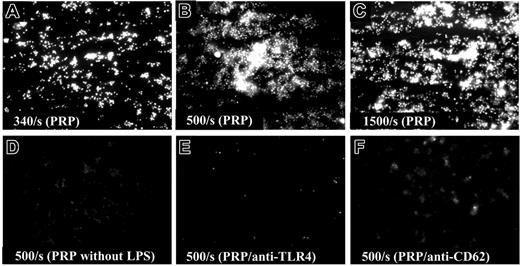
The interaction between HMEC-1-bound LPS and platelets at varying shear rates was studied using epifluorescence video microscopy, which showed increased binding at higher shear rates and that binding was TLR4 and CD62 dependent (Figure 5A-F). Attachment was negligible when WPs were used and no aggregates were visible (data not shown).
Relative roles of TLR4 and CD62 in platelet activation
The role of TLR4 and CD62 in LPS binding, signaling, and activation of platelets was studied in the 4 mouse groups with and without an anti-CD62 antibody (Table 6). Results indicate that TLR4 is essential for platelet activation because mutated mice did not express CD40L after stimulation with LPS. Partial reduction in CD40L expression was obtained after incubation of platelets from wild-type mice with anti-CD62, suggesting that CD62 is involved in LPS-induced platelet activation. Furthermore, LPS binding to platelets of C57BL/10ScN mutated mice was mediated by the formation of a complex between sCD14 and LPS because sCD14 was detected on these platelets only after incubation with LPS (median, 55%: range, 22.0-65.4, in comparison with platelets without LPS; median, 0.02%). Thus, sCD14 can bind to TLR413 and presumably even to CD62, but a resting platelet expresses TLR4, not CD62.
LPS and Stx are present on platelets from patients with EHEC infection
Platelets from 14 children treated at Lund University Hospital for EHEC-associated HUS were examined by immunofluorescence microscopy for LPS and Stx during the acute phase of disease and after recovery. O157LPS was detected on the platelets of 11 of 13 patients infected with E coli O157 (Table 7) but not after recovery (n = 5; patients 3, 4, 6, 11, 12). Stx1 was detected on the platelets from 10 patients, and Stx2 was detected on the platelets of 7 patients. Platelets from 5 patients bound both anti-Stx1 and anti-Stx2 to their platelets. Platelets from 12 of the children were positive for fibrinogen, indicating platelet activation, and platelets from 2 patients expressed CD62. After recovery (n = 5), no binding of any antibody was detected. Platelets from 5 pediatric controls were negative for O157LPS, Stx1, and Stx2. Results show that platelets from patients with EHEC-associated HUS carry bacterial components on their surfaces.
To determine whether bacterial components bind to platelets before HUS develops, platelets from 2 children treated at CHRMC for E coli O157:H7-associated HUS were examined. Platelets were available 1 and 3 days before HUS developed. LPS and Stx bound to platelets before HUS developed, and binding persisted when HUS developed in patient 16 (Table 7). Platelets from 3 children with uncomplicated EHEC infection (serotypes O157 [n = 2] and O177 [n = 1], all three strains producing Stx1 and Stx2), did not bind LPS, Stx, CD62, or fibrinogen antibodies (data not shown).
Discussion
LPS from E coli O157:H7 and other EHEC strains bind to and activate platelets by way of a novel TLR4/CD62 receptor complex. TLR4 is present on the surfaces of resting platelets; CD62 is not, but it contributes to platelet activation after LPS binding and signaling through TLR4. The interaction between LPS and human platelets leads to activation, as shown by expression of the activated GPIIb/IIIa receptor, CD40L, and by increased fibrinogen binding on platelets and the formation of aggregates in shear flow. LPS was detected on the platelets of children with EHEC-associated HUS, and binding was detected even before HUS developed but not on platelets from children with uncomplicated EHEC infection. We suggest that LPS binding might activate platelets and precipitate their consumption during HUS, which would contribute to thrombotic microangiopathy in target organs.
LPS binds myeloid cells through a receptor complex consisting of CD14, TLR4, and MD2. Certain nonmyeloid cells lacking membrane-bound CD14 respond to LPS in the presence of sCD14.13 This study demonstrated that the sCD14-LPS complex binds to platelets. Mice have sCD14,28 but CD14, after mediating binding to TLR4, does not appear to participate in platelet activation, as demonstrated in C3H/HeJ mice. sCD14-LPS binds to activated platelets in the absence of TLR4, indicating that binding to activated platelets may occur through TLR4 and CD62. The role of CD62 in platelet signaling appears to be secondary to TLR4 because LPS did not activate platelets or reduce platelet counts in C3H/HeJ mice. This indicates that primary platelet activation after LPS binding occurs through TLR4, leading to intracellular signaling, after which CD62 is exposed and contributes to further binding and activation. If TLR4 signaling does not occur, CD62 does not participate in LPS-induced platelet activation.
Thrombin stimulation, which activates platelets and leads to exposure of CD62 and TLR4 on their surfaces, increased LPS binding to platelets. The fact that patient platelets, in spite of activation (demonstrated by fibrinogen binding), showed little CD62 on their surfaces could be attributed to an interaction between LPS and CD62 that impeded binding of the anti-CD62 antibody. LPS binding was abrogated by anti-TLR4 and anti-CD62 antibodies, suggesting that these receptors act in concert to bind LPS. A steric interaction between the antibodies and their receptors could, however, not be excluded on the basis of the proximity of TLR4 and CD62 on the platelet surface. Mouse experiments using TLR4 mutants and an anti-CD62 antibody confirmed that both TLR4 and CD62 play roles in platelet activation.
LPS-induced platelet activation. (A) HMEC-1 cells were grown on glass slides and incubated with O157LPS for 1 hour at 37°C. Before perfusion, cells were washed and serum-free medium was added. Platelets were fluorescently labeled with quinacrine dihydrochloride (10 μM). PRP perfused over HMEC-1 incubated with O157LPS at lower shear rates (340 s-1) showed multiple small aggregates of attached platelets. (B) Attachment increased at higher shear rates (500 s-1). (C) Larger aggregates formed at 1500 s-1. (D) PRP perfused over HMEC-1 cells that were not incubated with O157LPS. Preincubation of PRP with anti-human TLR4 (E) or anti-CD62 (F) inhibited binding between platelets and cell-bound LPS.
LPS-induced platelet activation. (A) HMEC-1 cells were grown on glass slides and incubated with O157LPS for 1 hour at 37°C. Before perfusion, cells were washed and serum-free medium was added. Platelets were fluorescently labeled with quinacrine dihydrochloride (10 μM). PRP perfused over HMEC-1 incubated with O157LPS at lower shear rates (340 s-1) showed multiple small aggregates of attached platelets. (B) Attachment increased at higher shear rates (500 s-1). (C) Larger aggregates formed at 1500 s-1. (D) PRP perfused over HMEC-1 cells that were not incubated with O157LPS. Preincubation of PRP with anti-human TLR4 (E) or anti-CD62 (F) inhibited binding between platelets and cell-bound LPS.
In vitro and in vivo studies have previously shown that LPS derived from different Gram-negative bacteria can activate platelets. Lipid A increases intracellular calcium and induces activation of platelet protein kinase C.29,30 LPS from Proteus mirabilis activates the platelet secretory process,31 leading to the production of thromboxane A2,32 and it stimulates the adhesion of platelets to collagen.33 BALB/c mice and rats given intravenous injections of LPS from E coli O55:B5 or O127:B8 showed an accumulation of platelet aggregates in the lung and liver.34,35 Rat platelet counts decreased after infusion of O127LPS.36
The in vivo consequences of LPS binding were also examined in this study. LPS reduced platelet counts in TLR4 wild-type mice but not in the mutant strains. These results are consistent with recent studies.37,38 In a mouse model, LPS induced platelet thrombus formation in a TLR4-dependent manner.39 The reduced platelet counts in our study might have been a direct consequence of LPS binding to platelets, or might have been mediated by LPS binding to other cells, secondarily triggering platelet activation. Therefore, we tested the interaction between platelets and LPS bound to endothelium and found that platelets became activated and attached to the cells. In line with our results, a previous study showed that LPS approximated platelets to endothelium and reduced their rolling velocity.40 Results suggest that platelets are activated directly by LPS or by LPS bound to endothelium. The lower binding and absence of aggregation of WPs might have been caused by the lack of plasma components, such as fibrinogen, LBP, and sCD14.
Many factors may affect platelet activation in HUS, such as toxin-induced endothelial cell damage,41 increased shear flow in capillaries, release of platelet-activating chemokines from monocytes42 and endothelial cells43 in contact with Stx and LPS, and direct interaction between platelets and Stx and LPS. We and others10,44,45 have shown that Stx can bind to platelets directly and activate them. Stx and LPS are present on patient platelets and may induce direct platelet activation. We suggest that platelet activation in HUS may result from a combination of these stimulatory factors, which could contribute to the formation of thrombi in HUS.
EHECs are neither invasive nor bacteremic pathogens. Instead, bacterial virulence factors plausibly gain access to the circulation, thereby eliciting organ damage. Endotoxemia has been suggested but not proven in EHEC infection.6 A rapid serum IgG response to the O157LPS antigen during acute infection suggests that this molecule gains access to the circulation,46 and the concentration of LBP is increased in the plasma of children with E coli O157:H7 infection.47 We show that patient platelets carry LPS and that even platelets obtained before HUS develops were coated with LPS, indicating binding early in illness. Our in vitro data suggest that binding is not reversible during the first 2 hours. Given that platelets (at least in health) have a lifespan of 10 days and that thrombocytopenia develops during the initial phase of disease, we postulate that LPS binding to platelets may not be reversible. We can only hypothesize as to when during the course of infection endotoxemia occurs. Results from 2 pre-HUS patients indicate that LPS is present on platelet membranes before the development of HUS.
O157LPS appears to bind to human platelets more than the other EHEC LPSs studied. Variations between different LPS O-side chain multimer lengths from various E coli and other Gram-negative bacteria have not been specifically addressed in this study, although previous studies have shown that the length of the LPS side chain may influence biologic activity.48 The higher affinity for platelets of the O157LPS may partially explain the predominance of this serogroup as a cause of HUS. However, such attribution of virulence to a specific LPS must take into account a variety of considerations. First, most sporadic and epidemic E coli O157 infections do not result in HUS, suggesting that host factors, and probably additional bacterial factors, influence disease outcome. Second, the proportion of infections caused by other serogroups that progress to HUS is not well known because such infections are underdiagnosed. Therefore, we cannot state with certainty that the risk for HUS is, indeed, higher with EHEC that expresses the O157LPS. Third, pathogenic Shiga toxin-producing E coli have evolved by acquiring genes and chromosomal islands that encode myriad molecules other than LPS.49 Allelic variants of at least some of these loci are associated with infection outcome.50 It is most likely that the development of HUS is multifactorial at the microbial and the host levels, but our data suggest that specific LPSs might play some role in pathogenesis.
Prepublished online as Blood First Edition Paper, March 2, 2006; DOI 10.1182/blood-2005-08-3219.
Supported by grants from The Swedish Research Council (06X-14008, D.K.; 16X-07934 and 16BI-14577,C.S.); the Swedish Renal Foundation, Greta and Johan Kock Foundation, Inga and John Hains Foundation, Crown Princess Lovisa's Society for Child Care, Thelma Zoegas Foundation, Maggie Stephens Foundation, The Foundation for Fighting Blood Diseases, and the Magnus Bergvall Foundation (all to D.K.); the Sven Jerring Foundation (A.S., D.K.); Blood and Defense Network at Lund University (C.S., M.M., D.K.); Crafoord Foundation, Royal Physiographic Society in Lund, AlfredÖsterlund Foundation (M.M., D.K.); Swedish Foundation for Strategic Research/Micman (C.S.); National Institutes of Health (grant RO1-DK 52081, P.I.T.), and the Public Health Agency of Canada (R.J.).
A.S. designed and performed research, analyzed data, and wrote the paper. M.S. performed research and analyzed data. M.M. performed research, analyzed data, and contributed to writing the paper. C.S. designed research, analyzed data, and contributed to writing the paper. P.I.T. designed research, contributed reagents, and contributed to writing the paper. J.C.M. designed the research project in Seattle and contributed patient data and samples. S.L.W. designed the research project in Seattle and contributed patient data and samples. R.J. designed research and contributed reagents. D.K. designed research, analyzed data, and wrote the paper.
Presented in part at the Thirteenth Congress of the International Pediatric Nephrology Association, Adelaide, Australia, August 29 to September 2, 2004, and at the 5th International Symposium on Shiga Toxin (Verocytotoxin)-producing Escherichia coli Infections, Edinburgh, Scotland, June 8-11, 2003.51
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ann-Christine Sjögren and Maria Baumgarten for technical expertise, Ulf Sjöbring for providing access to epifluorescence video microscopy, and Rita Wallén and Eric Hallberg (Department of Cell and Organism Biology) and Lina Gefors (Jubileums Institute, Lund University) for assistance with electron microscopy. D.K. is the recipient of a clinical-experimental research fellowship from the Royal Swedish Academy of Sciences.