The efficacy of radioimmunotherapy (RIT) for patients with relapsed non-Hodgkin lymphoma (NHL) is limited by nonspecific delivery of radiation to normal tissues due to the long circulating half-life of radiolabeled anti-CD20 antibodies (Abs). Pretargeted RIT using a covalent conjugate of the 1F5 anti-CD20 Ab with streptavidin (SA) has been shown to augment the efficacy of RIT and decrease toxicity compared with a directly labeled 1F5 Ab. We have engineered a tetravalent singlechain 1F5 (scFv)4SA fusion protein and compared it to the 1F5-SA conjugate. Athymic mice bearing Ramos lymphoma xenografts received either the conjugate or fusion protein, followed 20 hours later by a biotin-N-acetyl-galactosamine clearing agent, followed 4 hours later by 111In-DOTA-biotin. After 24 hours, 11.4% ± 2.1% of the injected dose of radionuclide was present per gram of tumor (% ID/g) using 1F5 (scFv)4SA compared with 10.8% ± 2.5% ID/g with 1F5 Ab-SA. Superior tumor-to-normal organ ratios of radioactivity were consistently seen using the fusion protein compared with the chemical conjugate (eg, tumor-to-blood ratio > 65:1 after 48 hours with the fusion protein, but < 7:1 with the conjugate). More than 90% of lymphomabearing mice could be cured with minimal toxicity using either reagent followed by 1200 μCi (44.4 MBq) 90Y-DOTA-biotin. (Blood. 2006;108:328-336)
Introduction
Radioimmunotherapy (RIT) using anti-CD20 antibodies (Abs) conjugated to 131I or 90Y has shown promising efficacy and tolerable toxicity in many patients with indolent non-Hodgkin lymphoma (NHL).1-5 Recent data have suggested that improved efficacy can be achieved using standard nonmyeloablative doses of RIT with chemotherapy during early patient treatment encounters.6-8 In particular, outstanding results have been seen using conventional RIT as a single agent as frontline therapy for newly diagnosed patients.9 However, despite remission rates of 60% to 80% and complete response rates of 25% to 40% in multiply treated patients with NHL, most of these relapsed/refractory patients who receive nonmyeloablative doses of RIT subsequently relapse and the median progression-free survival rate is only about 1 year.10 The failure of conventional one-step RIT to completely eradicate lymphoma in these cases is presumably due to inadequate delivery of sufficient radiation since tumor-to-normal organ ratios of absorbed radioactivity are relatively low.11,12 One major obstacle limiting the efficacy of RIT is the protracted circulating half-life of conventional radiolabeled Abs, which necessitates nonspecific exposure of normal organs, particularly the bone marrow, to radioactivity. Pretargeted RIT (PRIT) is a strategy that may address this limitation and improve the therapeutic index by separating the localization of Ab to tumor sites from the delivery of the therapeutic radionuclide. One PRIT approach uses the high-affinity treptavidin (SA)-biotin system in which an Ab-SA conjugate and radioactive biotin are administered separately.13-16 The localization of the Ab-SA component to tumor is relatively slow and an unbound portion of the conjugate remains in circulation. However, because no radionuclide is attached, there are no toxic consequences. After maximal accumulation of Ab-SA conjugate in targeted tissues, a clearing agent (CA) is administered to remove circulating Ab-SA conjugate.17,18 Therapeutic radiobiotin is then administered and penetrates tumors rapidly because of its small size and binds with high affinity to the pretargeted Ab-SA conjugate. Excess radiobiotin is rapidly excreted by the kidney. This PRIT approach has been shown to improve the ratios of radiation delivered to tumors compared with normal organs in both preclinical and clinical models.17,19-28
Most pretargeting studies have employed chemically heterogeneous Ab-SA conjugates produced using heterobifunctional crosslinkers (eg, some Ab-SA conjugates have consisted of 80%-85% 1:1 Ab-SA conjugates, 5%-10% 1:2 Ab-SA conjugates, and 6%-10% molecules of higher molecular weight). In contrast, genetically engineered Ab-SA fusion proteins (FPs) are more homogeneous, more amenable to scale-up and approval by regulatory agencies, and more economical to produce. It has recently been demonstrated that a recombinant FP composed of an anti-CD20 single-chain Ab (scFv) and SA could be expressed at high levels in the periplasmic space of E coli.25 Additional fusion constructs targeting the CD25, EpCAM, and TAG72 antigens have also been expressed, purified in high yield, and investigated.18,24,29 These Ab-SA FPs spontaneously form tetramers, preserve the full antigen and biotin binding capabilities of both parent molecules, and bind more avidly to tumor cells than bivalent Ab-SA chemical conjugates.
In view of these compelling advantages, we engineered an anti-CD20 FP combining the single-chain Fv segment of the 1F5 murine anti-human CD20 Ab with SA to compare its biodistribution, pharmacokinetics, tumor localization index, and therapeutic efficacy with a corresponding anti-CD20 Ab-SA synthetic conjugate. We report here that the tetravalent FP localizes to tumors better than the chemical conjugate and is at least equivalent therapeutically.
Materials and methods
Cell lines
The CD20-expressing Ramos cell line (Burkitt lymphoma) and a hybridoma expressing a nonspecific IgG2, a negative control Ab, HB-8181, were obtained from American Type Culture Collection (Bethesda, MD). The 1F5 hybridoma cell line expressing the murine IgG2, an anti-human CD20 Ab, was a gift from Clay Siegall (Seattle Genetics, Seattle, WA). Cell lines were maintained using medium previously described.20 Cell viability exceeded 95% by trypan blue exclusion.
Antibodies
The 1F5 and HB8181 Abs were produced from the respective hybridomas using a hollow fiber bioreactor system in the monoclonal Ab production facility at the Fred Hutchinson Cancer Research Center (FHCRC; Seattle, WA).
Construction of a 1F5 (scFv)4SA fusion protein-expressing plasmid. The 1F5 heavy-chain (VH) and light-chain (VL) genes were cloned from the 1F5 hybridoma as published.30 The 1F5 scFvSA gene for the current study was engineered by fusing the scFv gene to the full-length genomic SA of Streptomyces avidinii as described.25,29 The resultant plasmid (A15-1-2) encoding the 1F5 scFvSA gene contained a 25-mer Gly4Ser linker25 between the VH and VL fragments. The SA gene was then joined to the scFv region using a GSGSA peptide linker. The FP was expressed from an IPTG-inducible lac promoter.
Expression and purification of 1F5 (scFv)4SA fusion protein. An E coli XL1 Blue (Stratagene, La Jolla, CA) transformant of the 1F5 scFvSA construct (A15-1-2) was grown in shaker flasks for qualitative expression of the FP and subsequently ina4L fermentor (BioFlo 3000; New Brunswick Scientific, Edison, NJ) using methods similar to those described.25 Cultures were induced with 0.1 mM IPTG and cells harvested after 44 hours. The cell paste was washed 3 times with PBS and the lysate purified by iminobiotin column chromatography.25 The eluted FP was treated with 20% DMSO for 2 hours to reduce aggregates, extensively dialyzed in PBS, filter-sterilized, formulated in 5% sorbitol, and stored at -80°C.
In vitro characterization of 1F5 (scFv)4SA fusion protein. The FP was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 4% to 12% Tris-glycine gels (Invitrogen, Carlsbad, CA) under nonreducing conditions. For immunoblot analysis, a goat anti-SA Ab (Vector Laboratories, Burlingame, CA) was used to detect the FP on a PVDF membrane (Invitrogen) using a peroxidase-conjugated F(ab')2 fragment of rabbit anti-goat IgG (Jackson ImmunoResearch, West Grove, PA) and a TMB substrate (Vector Laboratories). Size-exclusion high-performance liquid chromatography (HPLC) analysis was performed on a Zorbax GF-250 column (4.6 × 250 mm; Agilent, Palo Alto, CA) with a 20 mM sodium phosphate/0.5 M sodium chloride/15% DMSO/pH 6.8-7.0 mobile phase. Unpurified protein samples were quantified using a rhodaminebiotin assay as described.25 The concentration of the FP in the crude lysate was calculated using the molecular weight of the 1F5 FP as determined by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry. Competitive immunoreactivity was assessed by incubating 500 000 Ramos cells at 4°C for 1 hour with a mixture of 3 μg FITC-labeled 1F5 Ab and titered amounts of unlabeled 1F5 FP as a competing agent. Unlabeled 1F5 Ab or CC49 (scFv)4SA were used as positive and negative controls, respectively. Mean fluorescence was measured on a FACScan flow cytometer (Becton Dickinson Labware, Franklin Lakes, NJ). Biotin-binding capacity was determined by incubating the FP (100 μL, 1-2 nM/mL) with freshly diluted biotin-cyanocobalamin (10 μL, 2.16 mg/mL; Quanta BioDesign, Powell, OH) covered from light at room temperature for 30 minutes, followed by HPLC analysis. Quantification of the amount of unbound biotin-cyanocobalamin was obtained by comparison of the area under the biotin-cyanocobalamin peak at 362 nm with an unbound biotin standard curve.
Other fusion proteins and the 1F5 Ab-SA chemical conjugate
The anti-CD20 B9E9 (scFv)4SA (positive control) and the CC49 (scFv)4SA FP (negative control) were gifts from NeoRx (Seattle, WA). CC49(scFv)4SA recognizes the TAG 72 antigen expressed on most human adenocarcinomas, but not on lymphomas. The 1F5 Ab-SA covalent chemical conjugate (1F5 Ab-SA) was prepared as described previously.20
Radiolabeling
The 1F5 (scFv)4SA FP and 1F5 Ab-SA chemical conjugate were iodinated with either Na125IorNa131I (PerkinElmer, Boston, MA) by the chloramine T method, as previously published.20 111In and 90Y (PerkinElmer) radiolabeling of DOTA-biotin for PRIT or intact DOTA-1F5 Ab for conventional RIT was performed as described.20 Radiochemical purity was typically greater than 99% as determined by HPLC, and labeling efficiencies were greater than 93%. DOTA-biotin was synthesized as described.20
Immunoreactivity/avidity of Abs
The relative immunoreactivities for 1F5 Ab and 1F5 (scFv)4SA were investigated in a competitive binding assay that measured the binding of fluorescein-labeled 1F5 Ab to the Ramos cell line in the presence of various concentrations of unlabeled Ab. 1F5 Ab was labeled using fluorescein N-hydroxysuccinimide, and an optimized amount of this conjugate was mixed with serial dilutions (100 μg/mL) of 1F5 Ab standard or molar equivalents of 1F5 (scFv)4SA and incubated with 1 × 106 cells at 4°C for 30 minutes. After washing, samples were analyzed on a single-laser FACScan flow cytometer (Becton Dickinson Labware). After gating on single cells, the geometric mean fluorescence intensity was determined from a histogram plot of fluorescence. The concentration of competitor Ab required for 50% inhibition (IC50) of fluorescein-1F5 Ab binding was calculated using nonlinear regression analysis for one-site binding. Percent immunoreactivity was calculated according to the formula: [IC50 (scFv)4SA/IC50 Ab] × 100.
The immunoreactivities of radiolabeled reagents were also assessed by a cell-binding assay. 125I-labeled 1F5 Ab or 1F5 (scFv)4SA (100 ng/mL) was incubated with varying amounts of antigen (0.16 × 106-40 × 106 Ramos cells). Nonspecific binding was determined in the presence of excess cold Ab or FP (250 μg/mL). After incubation on a rotator for 2 hours at room temperature, bound and free Abs were separated by centrifugation through oil (1:1 dinonyl phthalate/dibutyl phthalate) and counted on a gamma counter. Immunoreactivity was calculated from Bmax determined from nonlinear regression analysis of a plot of bound (specific - nonspecific binding)/total (bound + free) versus cell concentration.31,32
Avidity was determined using saturation binding experiments that measure specific binding of radiolabeled Abs or (scFv)4SA (0.02 ng/mL-40 ng/mL) at equilibrium in the presence of excess antigen (107 cells). Nonspecific binding was determined in the presence of excess nonlabeled Ab or FP (250 μg/mL). Cell/Ab mixtures were incubated and centrifuged as described for immunoreactivity. The equilibrium dissociation constant (Kd) was calculated from nonlinear regression analysis of nM bound versus bound/free using immunoreactivity-adjusted Ab concentrations.32,33
Mouse studies
Female BALB/c nu/nu mice, age 6 to 8 weeks, were obtained from Animal Technologies (Kent, WA) and housed under protocols approved by the FHCRC Institutional Animal Care and Use Committee. Ramos cells (7 × 106) were injected subcutaneously in the right flank approximately 10 days prior to therapy to obtain lymphoma xenografts. Mice with similar, palpable tumors were chosen for the studies. For all pretargeting experiments, animals were placed on a biotin-deficient diet (Purina Mills, Richmond, IN) 4 to 5 days prior to treatment, and were maintained on the diet for 5 to 7 days following radiobiotin administration.
Pharmacokinetic and biodistribution studies. To assess whole-blood clearance, 1.4 nM of 1F5 (scFv)4SA (245 μg) and 1F5 Ab-SA (300 μg) were iodinated with either Na125I or Na131I and coinjected intraperitoneally into athymic mice. Blood radioactivity was measured by counting serially collected blood samples from the retro-orbital venus plexus in a gamma counter (Packard Cobra II gamma counter, Downers Grove, IL). For blood-clearance studies, a group of 5 mice were treated with an intraperitoneal injection of 1.4 nM (245 μg) 1F5 (scFv)4SA followed 20 hours later by a single intraperitoneal injection of a synthetic biotinylated CA (5.8 nM; 50 μg).21 Blood samples were counted for 125I/131I activity and corrected for 131I crossover into the 125I channel and for radioactive decay. An aliquot of the injectate was used to determine the injected dose. The mean values and standard deviations were plotted and blood half lives and areas under the curves (AUCs) calculated using Graphpad 4 Prism software (San Diego, CA).
For pretargeted biodistribution studies, groups of 5 mice with palpable xenografts were injected intraperitoneally with 1.4 nM of either B9E9 (scFv)4SA, 1F5 (scFv)4SA, the negative control CC49 (scFv)4SA, or 1F5 Ab-SA chemical conjugate. After 20 hours, 50 μg CA was administered intraperitoneally followed 4 hours later by 1.2 nM (1 μg) 111In-DOTA-biotin. Blood samples, tumors, and normal organs were obtained and counted for 111In activity as described previously.20
Therapy and toxicity studies. The therapeutic efficacy of the 1F5 Ab-SA chemical conjugate, 1F5 (scFv)4SA, and B9E9 (scFv)4SA were compared using various doses of 90Y-DOTA-biotin in groups of 10 mice bearing palpable subcutaneous Ramos xenografts. CC49 (scFv)4SA FP was used as a negative control. Mice were given either 1.4 nM (300 μg) 1F5 Ab-SA or 1.4 nM (245 μg) B9E9 or 1F5 (scFv)4SA, followed by 5.8 nM (50 μg) CA 20 hours later. A single dose of 1.2 nM (1 μg) DOTA-biotin labeled with 200, 400, 800, 1000, or 1200 μCi (7.4, 14.8, 29.6, 37, 44.4 MBq, respectively) 90Y-DOTA-biotin was administered 4 hours later.
In a second set of experiments, groups of 10 tumor-bearing mice were injected with either 1.4 nM 1F5 Ab-SA (300 μg), 1.4 nM 1F5 (scFv)4SA (245 μg), or 2.8 nM 1F5 (scFv)4SA (490 μg). Twenty hours later, 5.8 nM (50 μg) CA was administered to each animal, followed 4 hours later by 1.2 nM (1 μg) DOTA-biotin labeled with either 800 μCi or 1200 μCi 90Y (29.6 MBq and 44.4 MBq, respectively). Injections were given intraperitoneally in all therapy studies. Mice were assessed every other day for tumor volume measurements, weight change, and general appearance. Mice were euthanized if xenografts exceeded 10% of total body weight, caused obvious discomfort, or impaired ambulation.
Two separate toxicity experiments assessing leukocyte, hemoglobin, and platelet counts, plus aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatinine, and blood urea nitrogen (BUN) levels, were performed using mice treated as described earlier in this section using methods previously defined.20 Surviving mice were maintained for 12 months and serum again analyzed for AST, ALT, BUN, and creatinine to assess potential long-term kidney and liver toxicities.
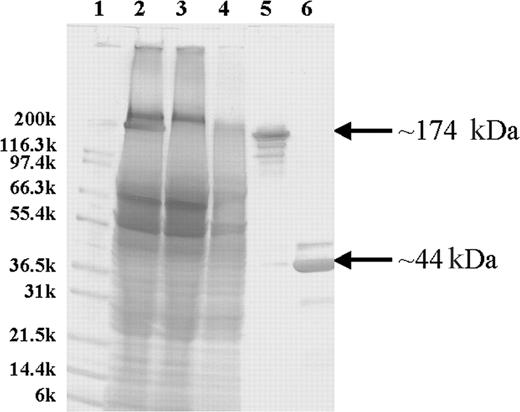
Purification and characterization of the 1F5 (scFv)4SA fusion protein. SDS-PAGE analysis of unpurified and iminobiotin-purified 1F5 (scFv)4SA with Coomassie Blue staining. The molecular weights in kilodaltons for Mark12 standards (Invitrogen) are indicated on the left (lane 1). Lane 2 contains a crude lysate from a culture of E coli-producing 1F5 (scFv)4SA. Lanes 3 and 4 contain the flow-through and wash fractions, respectively, from an iminobiotin purification column. Lanes 5 and 6 show the iminobiotin-purified FP either without boiling (lane 5) or denatured by boiling for 5 minutes (lane 6). The FP mass (174 kDa) and the monomer (44 kDa) are indicated on the right.
Purification and characterization of the 1F5 (scFv)4SA fusion protein. SDS-PAGE analysis of unpurified and iminobiotin-purified 1F5 (scFv)4SA with Coomassie Blue staining. The molecular weights in kilodaltons for Mark12 standards (Invitrogen) are indicated on the left (lane 1). Lane 2 contains a crude lysate from a culture of E coli-producing 1F5 (scFv)4SA. Lanes 3 and 4 contain the flow-through and wash fractions, respectively, from an iminobiotin purification column. Lanes 5 and 6 show the iminobiotin-purified FP either without boiling (lane 5) or denatured by boiling for 5 minutes (lane 6). The FP mass (174 kDa) and the monomer (44 kDa) are indicated on the right.
Results
Expression, purification, and biochemical characterization of 1F5 (scFv)4SA
An scFv fragment, made from VH and VL cDNAs encoding the immunoglobulin variable regions of the 1F5 Ab, was fused in-frame to the full-length SA gene of Streptomyces avidinii between its leader and mature-protein coding sequences to construct a 1F5 (scFv)4SA-expressing plasmid. The fusion gene encoded a mature protein of 422 amino acids with a calculated monomeric weight of 43 592 Da, resulting in a soluble tetramer in the periplasmic space of E coli with a molecular weight of 174 368 Da. One (scFv)4SA transformant (A15-1-2) was grown ina4L fermentor and expressed 100 mg/L of FP. The product was purified from homogenized cell extracts with a 59% yield on a single iminobiotin affinity column and an approximately 50% overall purification yield. SDS-PAGE analysis demonstrated 95% homogeneity of the FP after iminobiotin chromatography (Figure 1). The major protein band migrated at a position corresponding to the predicted tetramer molecular weight of 174 kDa. Additional minor isoforms were detected by Western blotting, but all bands resolved into a single species of Mr approximately 44 kDa when the FP was denatured by boiling before electrophoresis, consistent with a single protein entity dissociable into a homogeneous, monomeric subunit. Size-exclusion HPLC demonstrated that the purified FP exhibited a major peak with a retention time (8.25 minutes) appropriate for the tetramer and a minor peak (7.83 minutes) representing aggregated species (14%) with higher molecular weights. Aggregates were reduced to 1% after the purified FP was treated with 25% DMSO and analyzed by size-exclusion HPLC. MALDI-TOF mass spectroscopy of the FP established a molecular weight of 174 204 Da for the tetramer, in close agreement with the calculated mass of 174 379.16 Da for the most abundant isoform. Immunoreactivity was assessed by flow cytometry in a competitive assay with a fluorescence-labeled 1F5 Ab for binding to the CD20+ Ramos Burkitt lymphoma cell line. IC50 values of the competitive assay indicated that the tetravalent (scFv)4SA FP was about twice as immunoreactive as the divalent 1F5 Ab on a molar basis. Based on adjustment for its multivalency, the FP maintained full binding for the CD20 antigen. More precise quantification of the immunoreactivity and avidity of the 1F5 (scFv)4SA FP and the 1F5 Ab were obtained after radioiodinating each species and testing binding at various concentrations against serial dilutions of Ramos cells.25,31 These tests confirmed full retention of immunoreactivity and avidity by the 1F5 (scFv)4SA FP compared with the 1F5 Ab when tested on Ramos cells. Four experiments demonstrated an FP immunoreactivity of 87.8% ± 5.4%, compared with 76.0% ± 4.1% for the parent 1F5 Ab. The avidity of the tetravalent FP (Ka 3.26 ± 1.22 × 108 M-1) was superior to that of the bivalent Ab (1.31 ± 0.49 × 108 M-1) in 5 independent experiments (P = .027). For comparison, the B9E9 FP has been reported previously to have an immunoreactivity of 93% and an avidity of 0.80 × 108 M-1.25 The biotin-binding capacity of 1F5 (scFv)4SA was assessed by a cyanocobalimin-biotin HPLC assay, revealing an average of 4 of 4 possible biotin-binding sites per molecule of the FP, identical to recombinant SA (data not shown).
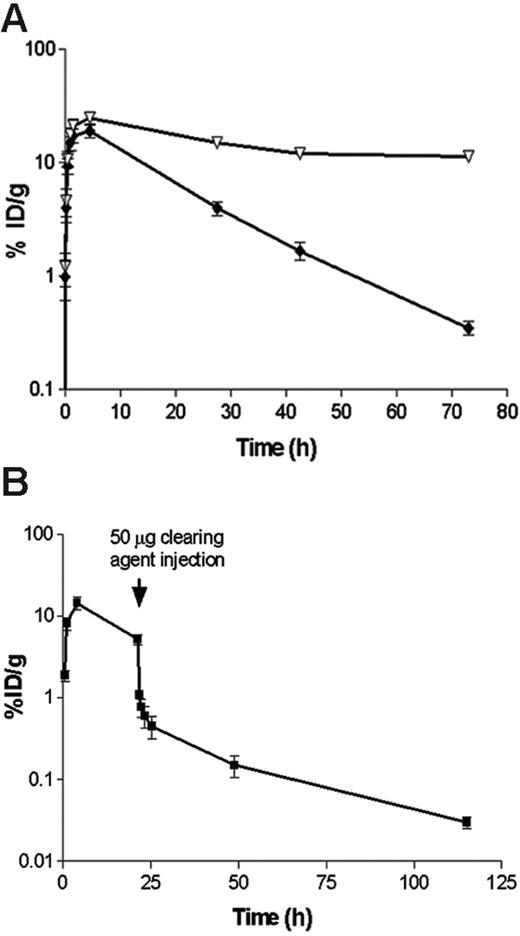
Pharmacokinetic and blood-clearance studies. (A) Whole-blood clearance of 1.4 nM (300 μg) 131I-labeled 1F5 Ab-SA chemical conjugate (▿) or 1.4 nM (245 μg) 125I-labeled 1F5 (scFv)4SA, each injected intraperitoneally into athymic BALB/c mice (n = 5/group). (B) The effect of a biotinylated polymeric, N-acetyl-galactosamine-containing CA, on circulating 125I-1F5 (scFv)4SA FP. 125I-1F5 (scFv)4SA (1.4 nM) was injected intraperitoneally into 5 BALB/c athymic mice at time 0 hours. The CA (50 μg; 5.8 nM intraperitoneally) was injected 20 hours after the labeled FP. In each experiment, serial blood samples were obtained from the retro-orbital venous plexus at the times indicated after the injection of each Ab-SA construct and analyzed by gamma counting. Results are representative of 3 experiments.
Pharmacokinetic and blood-clearance studies. (A) Whole-blood clearance of 1.4 nM (300 μg) 131I-labeled 1F5 Ab-SA chemical conjugate (▿) or 1.4 nM (245 μg) 125I-labeled 1F5 (scFv)4SA, each injected intraperitoneally into athymic BALB/c mice (n = 5/group). (B) The effect of a biotinylated polymeric, N-acetyl-galactosamine-containing CA, on circulating 125I-1F5 (scFv)4SA FP. 125I-1F5 (scFv)4SA (1.4 nM) was injected intraperitoneally into 5 BALB/c athymic mice at time 0 hours. The CA (50 μg; 5.8 nM intraperitoneally) was injected 20 hours after the labeled FP. In each experiment, serial blood samples were obtained from the retro-orbital venous plexus at the times indicated after the injection of each Ab-SA construct and analyzed by gamma counting. Results are representative of 3 experiments.
Blood clearance of 1F5 (scFv)4SA fusion protein
Blood clearance studies were conducted in female BALB/c athymic mice to assess the pharmacokinetic differences between 1F5 (scFv)4SA and 1F5 Ab-SA (Figure 2A). Equimolar doses (1.4 nM) of 131I-labeled conjugate and 125I-labeled FP were coinjected intraperitoneally and blood samples were serially drawn and counted for radioactivity. Blood concentrations of both reagents peaked 4 hours after injection with similar blood levels (24.5% ± 3.3% injected dose of absorbed radionuclide/gram [ID/g] for the chemical conjugate and 19.0% ± 5.3% ID/g for the FP). The FP had a blood-clearance half-life (t1/2 β) of 9.1 hours compared with 25 hours for the chemical conjugate. Area-under-the-curve (AUC) calculations demonstrated a 2- to 3-fold faster clearance of the FP than of the Ab-SA chemical conjugate (AUC 5965 pM/mL vs 15 260 pmol/mL, respectively). Despite the faster clearance of the FP from the blood compared with the covalent conjugate, the FP was still cleared from the circulation too slowly for optimal RIT without the use of a CA. In 3 separate experiments, a single 5.8-nM dose of a biotinylated polymeric, N-acetyl-galactosamine CA, was given intraperitoneally to athymic mice 20 hours after 125I-1F5 (scFv)4SA administration. The results demonstrated that the CA rapidly removed more than 90% of circulating 125I-1F5 (scFv)4SA from the blood within 1 hour, with a drop in whole-blood concentration from 8.1% ± 2.8% ID/g to 0.77% ± 0.38% ID/g (Figure 2B).
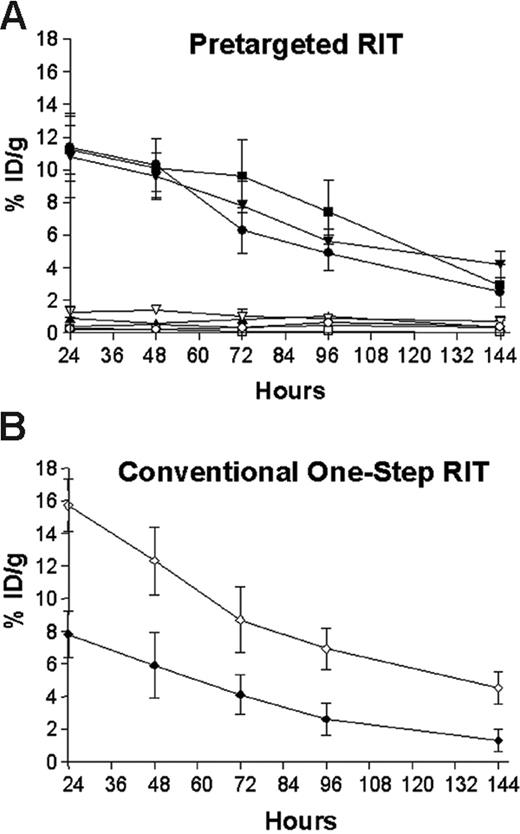
Biodistributions of radioactivity in blood and tumors of athymic mice bearing Ramos xenografts who were injected with either 1F5 (scFv)4SA fusion protein, B9E9 (scFv)4SA fusion protein, 1F5 Ab-SA chemical conjugate, CC49 (scFv)4SA fusion protein, or directly labeled conventional 111In-DOTA-1F5 Ab. Mice in pretargeted groups (A) were injected with 1.4 nM of each unlabeled construct, followed 20 hours later by 5.8 nM CA, and 4 hours after that by 1.2 nM 111In-DOTA-biotin. In the directly labeled group (B), mice were injected with 1.4 nM of conventional trace-labeled 111In-DOTA-1F5 Ab at time 0 hours. Groups of 5 mice were euthanized 24, 48, 96, and 144 hours after injection of radiobiotin or 111In-DOTA-1F5 Ab. The radioactivity in blood and tumors were quantified by gamma counting, corrected for decay, and expressed as the % ID/g of tissue. 1F5 (scFv)4SA fusion protein (•, tumor; ○, blood), B9E9 (scFv)4SA fusion protein (▪, tumor; □, blood), 1F5 Ab-SA chemical conjugate (▾, tumor; ▿, blood), CC49 (scFv)4SA fusion protein (▴, tumor; ▵, blood), directly labeled conventional 111In-DOTA-1F5 Ab (♦, tumor; ⋄, blood).
Biodistributions of radioactivity in blood and tumors of athymic mice bearing Ramos xenografts who were injected with either 1F5 (scFv)4SA fusion protein, B9E9 (scFv)4SA fusion protein, 1F5 Ab-SA chemical conjugate, CC49 (scFv)4SA fusion protein, or directly labeled conventional 111In-DOTA-1F5 Ab. Mice in pretargeted groups (A) were injected with 1.4 nM of each unlabeled construct, followed 20 hours later by 5.8 nM CA, and 4 hours after that by 1.2 nM 111In-DOTA-biotin. In the directly labeled group (B), mice were injected with 1.4 nM of conventional trace-labeled 111In-DOTA-1F5 Ab at time 0 hours. Groups of 5 mice were euthanized 24, 48, 96, and 144 hours after injection of radiobiotin or 111In-DOTA-1F5 Ab. The radioactivity in blood and tumors were quantified by gamma counting, corrected for decay, and expressed as the % ID/g of tissue. 1F5 (scFv)4SA fusion protein (•, tumor; ○, blood), B9E9 (scFv)4SA fusion protein (▪, tumor; □, blood), 1F5 Ab-SA chemical conjugate (▾, tumor; ▿, blood), CC49 (scFv)4SA fusion protein (▴, tumor; ▵, blood), directly labeled conventional 111In-DOTA-1F5 Ab (♦, tumor; ⋄, blood).
Comparative biodistributions of 111Indium in tumor-bearing mice pretargeted with either anti-CD20 (scFv)4SA or 1F5 Ab-SA or treated with directly labeled 111In-DOTA-1F5 Ab
Groups of 5 mice were injected intraperitoneally with either 1.4 nM of directly labeled 111In-DOTA-1F5 Ab, 1F5 (scFv)4SA, 1F5 Ab-SA, B9E9 (scFv)4SA (positive control), or CC49 (scFv)4SA (negative control). Mice receiving the 111In-DOTA-1F5 Ab were also coinjected with 400 μg of a nonspecific IgG2a Ab, HB-8181, to prevent nonspecific binding of the 1F5 Ab to Fc-receptors in the spleen and marrow.17 In PRIT studies, 5.8 nM (50 μg) CA was injected 20 hours after delivery of each FP or conjugate, followed 4 hours later by 1.2 nM 111In-DOTA-biotin. Groups of 5 mice were killed 24, 48, 72, 96, and 144 hours following 111In-DOTA-1F5 Ab or 111In-DOTA-biotin administration (Figure 3). Similar biodistributions of 111In-DOTA-biotin were obtained in tumors and blood following PRIT (Figure 3A) employing 1F5 (scFv)4SA, B9E9 (scFv)4SA, and 1F5 Ab-SA. After 24 hours, peak 111In-DOTA-biotin levels were 11.4% ± 2.1% ID/g with 1F5 (scFv)4SA, 10.8% ± 2.5% ID/g with 1F5 Ab-SA, and 11.2% ± 1.5% ID/g with B9E9 (scFv)4SA. The biodistributions of radioactivity in normal organs were similar with each pretargeted anti-CD20 construct and with the nonspecific CC49 (scFv)4SA negative control, ranging from less than 1% ID/g in colon to less than 2.5% ID/g in kidney (data not shown). However, circulating blood levels of 111In-DOTA-biotin after 24 hours were notably higher in mice receiving 1F5 Ab-SA (1.6% ± 0.2% ID/g), compared with either B9E9 (scFv)4SA (0.2% ± 0.1% ID/g) or 1F5 (scFv)4SA (0.3% ± 0.1% ID/g). At all timepoints, control animals injected with the nonbinding CC49 (scFv)4SA had negligible tumor uptake of radioactivity (0.9% ± 0.1% ID/g), demonstrating the specificity of targeting.
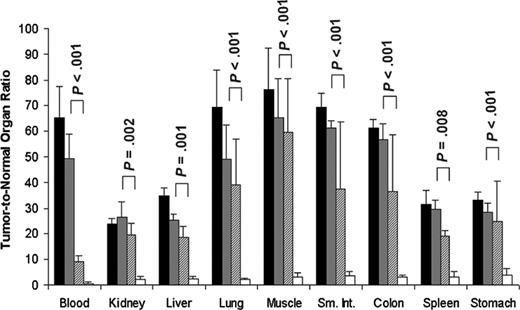
Tumor-to-normal organ ratios for conventional directly labeled 111In-DOTA-1F5 Ab or 111In-DOTA biotin pretargeted with B9E9 (scFv)4-SA fusion protein, 1F5 (scFv)4-SA fusion protein, or 1F5 Ab-SA chemical conjugate. Mice were treated as described in Figure 3. Tumor-to-normal organ ratios of administered 111In-DOTA-1F5 or 111In-DOTA biotin are shown for the 24-hour time point after injection of radioactivity. 1F5 (scFv)4SA fusion protein (▦), B9E9 (scFv)4SA fusion protein (▪), 1F5 Ab-SA chemical conjugate (▨), directly labeled conventional 111In-DOTA-1F5 Ab (□). P values represent differences between the 1F5 (scFv)4-SA fusion protein and directly labeled DOTA-1F5 Ab.
Tumor-to-normal organ ratios for conventional directly labeled 111In-DOTA-1F5 Ab or 111In-DOTA biotin pretargeted with B9E9 (scFv)4-SA fusion protein, 1F5 (scFv)4-SA fusion protein, or 1F5 Ab-SA chemical conjugate. Mice were treated as described in Figure 3. Tumor-to-normal organ ratios of administered 111In-DOTA-1F5 or 111In-DOTA biotin are shown for the 24-hour time point after injection of radioactivity. 1F5 (scFv)4SA fusion protein (▦), B9E9 (scFv)4SA fusion protein (▪), 1F5 Ab-SA chemical conjugate (▨), directly labeled conventional 111In-DOTA-1F5 Ab (□). P values represent differences between the 1F5 (scFv)4-SA fusion protein and directly labeled DOTA-1F5 Ab.
All 3 anti-CD20 pretargeting constructs had superior biodistributions compared with conventional RIT employing directly radiolabeled 111In-DOTA-1F5 whole Ab (Figure 3B). In contrast to the PRIT biodistribution experiments, only 7.8% ± 1.4% ID/g was present in tumor after 24 hours using 111In-DOTA-1F5 Ab, decreasing to 5.9% ± 2.0% ID/g after 48 hours, and 4.1% ± 1.2% ID/g of tumor after 72 hours. The amount of radioactivity remained high in the blood for prolonged periods following administration of the 111In-DOTA-1F5 Ab, with 15.7% ± 1.6% ID/g, 12.3% ± 2.1% ID/g, and 8.7% ± 2.0% ID/g in blood after 24, 48, and 72 hours, respectively. PRIT using either 1F5 (scFv)4SA, B9E9 (scFv)4SA, or the 1F5 Ab-SA yielded favorable tumor-to-normal organ ratios at all timepoints; however, ratios were most favorable using the pretargeted FPs, due to their more rapid clearance from blood and superior tumor retention. Using the pretargeting approach with 1F5 (scFv)4SA, the tumor-to-normal organ ratios after 24 hours varied from a high of about 50:1 in blood to the lowest being 26:1 in kidney (Figure 4). In contrast, the tumor-to-blood ratio was only 9:1 after 24 hours using pretargeted 1F5 Ab-SA and the tumor-to-kidney ratio was 19:1. The pretargeting approach resulted in superior biodistributions with both FPs and the chemical conjugate compared with conventional radiolabeled 1F5 Ab, which yielded a tumor-to-blood ratio of 0.5:1 and a tumor-to-kidney ratio of only 2.2:1 after 24 hours.
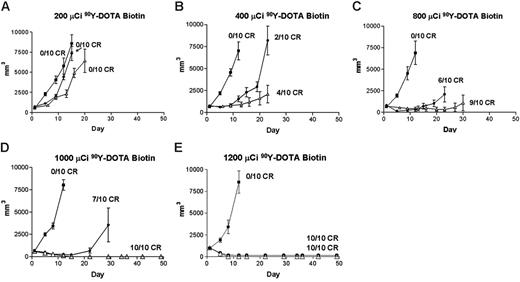
Regression of lymphoma xenografts after PRIT comparing anti-CD20 B9E9 (scFv)4SA fusion protein and 1F5 Ab-SA chemical conjugate. Athymic BALB/c mice bearing Ramos lymphoma xenografts were injected intraperitoneally with either 1.4 nM B9E9 (scFv)4SA (•) or 1F5 Ab-SA (▵), followed 20 hours later by 5.8 nM CA, and 4 hours after that with (A) 200, (B) 400, (C) 800, (D) 1000, or (E) 1200 μCi (7.4, 14.8, 29.6, 37, or 44.4 MBq, respectively) 90Y-DOTA-biotin. Tumor volume curves are truncated at the time of euthanasia of the first mouse in each group. Control mice bearing xenograft tumors were treated with pretargeted CC49 (scFv)4SA (▪).
Regression of lymphoma xenografts after PRIT comparing anti-CD20 B9E9 (scFv)4SA fusion protein and 1F5 Ab-SA chemical conjugate. Athymic BALB/c mice bearing Ramos lymphoma xenografts were injected intraperitoneally with either 1.4 nM B9E9 (scFv)4SA (•) or 1F5 Ab-SA (▵), followed 20 hours later by 5.8 nM CA, and 4 hours after that with (A) 200, (B) 400, (C) 800, (D) 1000, or (E) 1200 μCi (7.4, 14.8, 29.6, 37, or 44.4 MBq, respectively) 90Y-DOTA-biotin. Tumor volume curves are truncated at the time of euthanasia of the first mouse in each group. Control mice bearing xenograft tumors were treated with pretargeted CC49 (scFv)4SA (▪).
Pretargeted radioimmunotherapy with anti-CD20 fusion proteins and conjugates
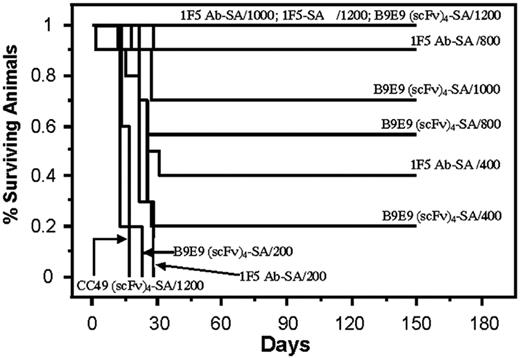
The therapeutic efficacies of the various PRIT reagents were compared in athymic mice bearing Ramos xenografts after injection of CA and 90Y-DOTA-biotin. Mice bearing palpable tumors were injected intraperitoneally with 1.4 nM 1F5 Ab-SA, B9E9 (scFv)4SA, or CC49 (scFv)4SA. CA was injected 20 hours later followed 4 hours later by 200, 400, 800, 1000, or 1200 μCi (7.4, 14.8, 29.6, 37, or 44.4 MBq, respectively) 90Y-DOTA-biotin in groups of 10 mice each (Figure 5). Xenografts grew exponentially in all negative control groups at all doses of 90Y-DOTA-biotin, including untreated mice, mice treated with 90Y-DOTA-biotin alone, and mice receiving CC49 (scFv)4SA followed by 90Y-DOTA-biotin, necessitating euthanasia by 15 days after treatment (Figure 6). Mice treated with B9E9 (scFv)4SA followed by 200 μCi 90Y-DOTA-biotin exhibited tumor growth rates similar to the negative control groups (Figure 5A), with required euthanasia by day 20 (Figure 6). The 1F5 Ab-SA group receiving 200 μCi (7.4 MBq) 90Y-DOTA-biotin showed a transient initial delay in tumor growth (Figure 5A), but all mice required euthanasia 27 to 30 days after treatment (Figure 6). Tumor xenograft growth was significantly slower in mice treated with B9E9 (scFv)4SA (2 of 10 complete remissions [CRs]) or 1F5 Ab-SA (4 of 10 CRs) followed by 400 μCi (14.8 MBq) 90Y-DOTA-biotin compared with the negative control groups (0 of 10 CRs; Figure 5B). Xenografts disappeared in 9 of 10 mice treated with 1F5 Ab-SA followed by 800 μCi (29.6 MBq) 90Y-DOTA-biotin and in all 10 mice treated with 1F5 Ab-SA and 1000 μCi (37 MBq) 90Y-DOTA-biotin (Figure 5C-D, respectively). By comparison, CRs were obtained in 4 of 10 mice treated with B9E9 (scFv)4SA and 800 μCi (29.6 MBq) 90Y-DOTA-biotin, and in 6 of 10 mice treated with 1000 μCi (37 MBq) 90Y-DOTA-biotin (Figure 5C-D, respectively). All mice receiving either B9E9 (scFv)4SA or 1F5 Ab-SA achieved CRs with 1200 μCi 90Y-DOTA-biotin by day 12 (Figure 5E). All CRs were durable for the full time course of the study (> 150 days), suggesting that these mice were cured (Figure 6).
Analysis of cumulative survival of mice bearing Ramos lymphoma xenografts treated with PRIT using anti-CD20 B9E9 (scFv)4SA fusion protein and 1F5 Ab-SA chemical conjugate. Groups of 10 mice bearing Ramos tumor xenografts were treated as described in Figure 5 and analyzed for survival as a function of time. Treatment groups included mice treated with 1.4 nM of either B9E9 (scFv)4SA, control CC49 (scFv)4SA, or 1F5 Ab-SA, followed 20 hours later by 5.8 nM CA, and 4 hours after that with 200, 400, 800, 1000, or 1200 μCi (7.4, 14.8, 29.6, 37, or 44.4 MBq, respectively) 90Y-DOTA-biotin. Survival curves in this figure correspond to treatment groups designated in Figure 5.
Analysis of cumulative survival of mice bearing Ramos lymphoma xenografts treated with PRIT using anti-CD20 B9E9 (scFv)4SA fusion protein and 1F5 Ab-SA chemical conjugate. Groups of 10 mice bearing Ramos tumor xenografts were treated as described in Figure 5 and analyzed for survival as a function of time. Treatment groups included mice treated with 1.4 nM of either B9E9 (scFv)4SA, control CC49 (scFv)4SA, or 1F5 Ab-SA, followed 20 hours later by 5.8 nM CA, and 4 hours after that with 200, 400, 800, 1000, or 1200 μCi (7.4, 14.8, 29.6, 37, or 44.4 MBq, respectively) 90Y-DOTA-biotin. Survival curves in this figure correspond to treatment groups designated in Figure 5.
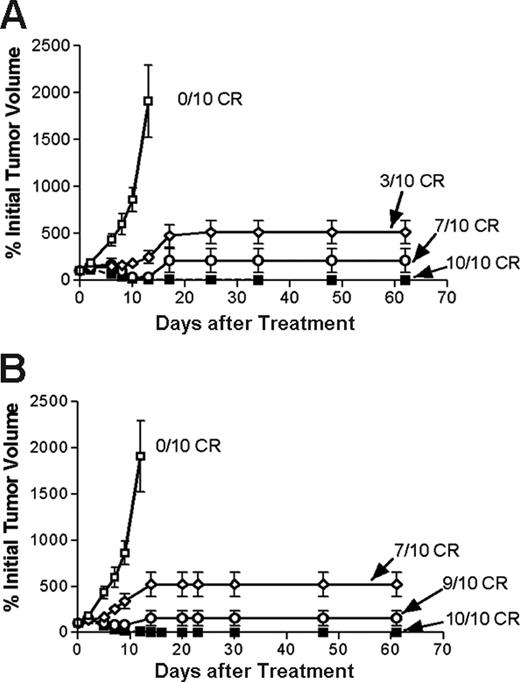
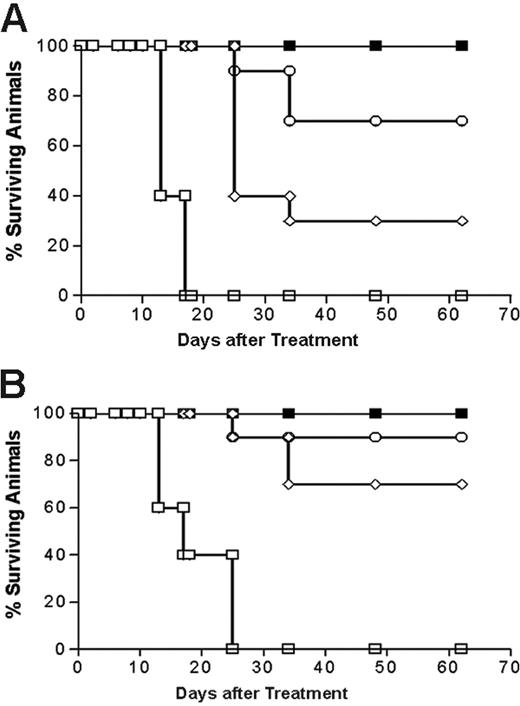
Next, the therapeutic efficacies of PRIT using 1.4 nM 1F5 Ab-SA and 1.4 nM or 2.8 nM 1F5 (scFv)4SA followed by either 800 μCi or 1200 μCi of 90Y-DOTA-biotin were directly compared in lymphoma-bearing mice. The escalated dose (2.8 nM) of 1F5 (scFv)4SA was studied to compensate for the 2- to 3-fold more rapid clearance of the FP from the circulation, based on AUC calculations noted in “Blood clearance of 1F5 (scFV)4 SA fusion protein.” Control groups of 10 mice each received 800 μCi (29.6 MBq) or 1200 μCi (44.4 MBq) 90Y-DOTA-biotin alone or 1.4 nM CC49 (scFv)4SA followed by CA and 800 μCi (29.6 MBq) or 1200 μCi (44.4 MBq) 90Y-DOTA-biotin. All control mice exhibited exponential xenograft growth, necessitating euthanasia by day 13 (Figure 7). Durable CRs were obtained in 3 of 10 animals treated with 1.4 nM 1F5 (scFv)4SA followed by 800 μCi (29.6 MBq) 90Y-DOTA-biotin and in 7 of 10 animals receiving 1.4 nM 1F5 (scFv)4SA and 1200 μCi (44.4 MBq) 90Y-DOTA-biotin (Figure 7B). When the dose of 1F5 (scFv)4SA was increased to 2.8 nM to compensate for its shorter half-life, the number of CRs increased to 7 of 10 treated with 800 μCi (29.6 MBq) 90Y-DOTA-biotin and 9 of 10 receiving 1200 μCi (44.4 MBq)90Y-DOTA-biotin (Figure 7B). CRs were seen by day 10 in all 10 mice treated with 1.4 nM 1F5 Ab-SA followed by either 800 μCi (29.6 MBq) or 1200 μCi (44.4 MBq) of radiobiotin. All mice achieving CR remained tumor-free for the entire observation period (> 150 days; Figure 8).
Regression of lymphoma xenografts after PRIT comparing anti-CD20 1F5 (scFv)4SA fusion protein and 1F5 Ab-SA chemical conjugate. Athymic BALB/c mice bearing Ramos lymphoma xenografts were injected intraperitoneally with either 1.4 (⋄) or 2.8 (○) nM 1F5 (scFv)4SA or 1F5 Ab-SA (▪), followed 20 hours later by 5.8 nM CA, and 4 hours after that with (A) 800 or (B) 1200 μCi (29.6 or 44.4 MBq, respectively) 90Y-DOTA-biotin. Control mice bearing xenograft tumors were treated with pretargeted CC49 (scFv)4SA (□). Tumor volume curves though day 60 incorporate the tumor size determined at the time of euthanasia.
Regression of lymphoma xenografts after PRIT comparing anti-CD20 1F5 (scFv)4SA fusion protein and 1F5 Ab-SA chemical conjugate. Athymic BALB/c mice bearing Ramos lymphoma xenografts were injected intraperitoneally with either 1.4 (⋄) or 2.8 (○) nM 1F5 (scFv)4SA or 1F5 Ab-SA (▪), followed 20 hours later by 5.8 nM CA, and 4 hours after that with (A) 800 or (B) 1200 μCi (29.6 or 44.4 MBq, respectively) 90Y-DOTA-biotin. Control mice bearing xenograft tumors were treated with pretargeted CC49 (scFv)4SA (□). Tumor volume curves though day 60 incorporate the tumor size determined at the time of euthanasia.
Analysis of cumulative survival of mice bearing Ramos lymphoma xenografts treated with PRIT using anti-CD20 1F5 (scFv)4SA fusion protein and 1F5 Ab-SA chemical conjugate. Groups of 10 mice bearing approximately 100 mm3Ramos tumor xenografts were treated as described in Figure 7 and analyzed for survival as a function of time. Treatment groups included mice treated with 1.4 (⋄) and 2.8 (○) nM of 1F5 (scFv)4SA, 1.4 nM of control CC49 (scFv)4SA (□), or 1.4 nM of 1F5 Ab-SA chemical conjugate (▪), followed 20 hours later by 5.8 nM CA, and 4 hours after that with (A) 800 or (B) 1200 μCi (29.6 or 44.4 MBq, respectively) 90Y-DOTA-biotin. Survival curves in this figure correspond to treatment groups designated in Figure 7.
Analysis of cumulative survival of mice bearing Ramos lymphoma xenografts treated with PRIT using anti-CD20 1F5 (scFv)4SA fusion protein and 1F5 Ab-SA chemical conjugate. Groups of 10 mice bearing approximately 100 mm3Ramos tumor xenografts were treated as described in Figure 7 and analyzed for survival as a function of time. Treatment groups included mice treated with 1.4 (⋄) and 2.8 (○) nM of 1F5 (scFv)4SA, 1.4 nM of control CC49 (scFv)4SA (□), or 1.4 nM of 1F5 Ab-SA chemical conjugate (▪), followed 20 hours later by 5.8 nM CA, and 4 hours after that with (A) 800 or (B) 1200 μCi (29.6 or 44.4 MBq, respectively) 90Y-DOTA-biotin. Survival curves in this figure correspond to treatment groups designated in Figure 7.
Toxicity
Toxicities were assessed in groups of 5 mice receiving 1200 μCi (44.4 MBq) 90Y-DOTA-biotin pretargeted with 1.4 nM of either 1F5 Ab-SA, 1F5 (scFv)4SA, B9E9 (scFv)4SA, or CC49 (scFv)4SA, and cleared from blood with 5.8 nM CA. Mice without tumors were used so that serial blood collections could be obtained without euthanizing mice because of xenograft growth. In addition, toxicities were evaluated after administration of 200 μCi (7.4 MBq) of directly labeled 90Y-DOTA-1F5 Ab. Higher doses of directly labeled 90Y-DOTA-1F5 were not studied since they were lethal in 100% of mice.17 Weekly leukocyte, platelet, and hemoglobin values were obtained using blood sampled from the retro-orbital venous plexus (Table 1). Leukocyte counts in all groups reached a nadir 7 to 14 days after radiobiotin. Mice treated with 1200 μCi (44.4 MBq) 90Y-DOTA-biotin after the CC49 (scFv)4SA produced a leukocyte nadir (51% ± 39% of initial leukocyte count) similar to 1F5 Ab-SA (39% ± 54%), 1F5 (scFv)4SA (48% ± 36%), and B9E9 (scFv)4SA (41% ± 27%). In contrast, hemoglobin values and platelet levels did not decrease in any pretargeted group. Mice injected with 200 μCi (7.4 MBq) conventional 90Y-DOTA-1F5 Ab exhibited more profound decrements in the leukocyte count (76% ± 40% by day 7) as well as anemia (48% ± 17% drop in hemoglobin by day 14) and thrombocytopenia (43% ± 9% decrement in platelet count by day 7). All mice in the conventional RIT group died of toxicity by day 30.
In a separate study using the same treatment protocol, blood was sampled weekly for AST, ALT, creatinine, and BUN levels. There were no significant differences in BUN, creatinine, or transaminase levels detected between groups of mice treated with either directly labeled 200 μCi (7.4 MBq) 90Y-DOTA-1F5 Ab or anti-CD20 fusion protein or 1F5-SA chemical conjugate followed by 1200 μCi (44.4 MBq) 90Y-DOTA-biotin. All chemistry values remained stable in all treatment groups at all time points up to 1 year, suggesting the absence of renal or hepatic toxicity even in pretargeted animals receiving 1200 μCi (44.4 MBq) 90Y-DOTA-biotin.
Discussion
The recent development of radiolabeled Abs presents a unique opportunity to treat hematologic malignancies by delivering high doses of radiation specifically to tumor sites while sparing normal organs. Radiolabeled Abs using either 131Ior 90Y have been used by many groups at nonmyeloablative doses to target a variety of NHL cell-surface antigens other than CD20 including CD19, CD22, CD37, and HLA class II molecules.34-36 The most promising trials have documented the safety and efficacy of 131I-tositumomab and 90Y-ibritumomab targeting CD20 for relapsed patients with NHL.1-5 However, despite remission rates of 60% to 80% and CR rates of 25% to 40% with these anti-CD20 radioimmunoconjugates, most patients relapse with a median progression-free survival of only 6 to 12 months.10 One major obstacle limiting the efficacy of RIT is the relatively protracted circulating half-life of conventional radio-labeled Abs that results in nonspecific exposure of normal organs to radioactivity. In this report, we have attempted to overcome this limitation and improve the target-to-nontarget ratios of absorbed radiation doses using a PRIT method that permits rapid clearance of the targeting Ab construct from the blood. Several studies in solid tumor and hematologic malignancies have supported the superiority of PRIT compared with conventional RIT because of the more rapid uptake of radiolabel in the tumor, more rapid blood clearance of radioactivity, and improvement in tumor-to-blood and tumor-to-normal organ radioactivity ratios.17-24,27,37-40 Several pre-targeting methods have been developed including (1) bispecific Abs that target a specific target site and possess a hapten-binding site41 ; (2) biotinylated Abs that bind to SA42 ; and (3) Abs conjugated to SA or avidin that bind to biotin.21,43 While the optimal PRIT strategy remains to be elucidated, we have chosen to pursue the 2-step Ab-SA approach.
Although PRIT offers striking advantages, some caveats to the clinical implementation of this strategy should also be acknowledged. PRIT requires multiple injections of reagents at defined time intervals and necessitates the validation of the safety of each step. The immunogenicity of SA may limit the ability to administer multiple cycles of therapy. Moreover, endogenous biotin and serum biotinidases may limit the therapeutic success of radiolabeled biotin binding to SA, although the presence of endogenous biotin and serum biotinidases have not been serious impediments to the success of this approach.28,44
Most Ab-SA pretargeting studies have used chemically synthesized covalent conjugates that deliver at least 5-fold higher doses of absorbed radiation to tumor sites compared with conventional RIT, while at the same time exposing normal tissues to lower doses of irradiation. Chemically cross-linked conjugates between whole Abs and SA, however, are invariably mixtures of heterogeneous protein species that lack uniformity and potentially impact the biotin-binding capacity of the SA molecule adversely. Recently, genetically engineered tetravalent single-chain Fv fusion constructs have been developed to achieve better biochemical uniformity for PRIT.25,29 These FPs have several distinct advantages. The absence of the Fc portion present in intact Abs results in the more rapid systemic clearance of the FPs compared with the first generation covalent whole Ab-SA conjugates and minimizes uptake of FPs in specialized Ab reservoirs.45 Recombinant FPs also have the advantage of spontaneous tetramer formation, allowing amplification of the amount of radiation delivered to tumor cells. Moreover, genetically engineered FPs can be expressed at high levels in the periplasmic space of E coli, and are thus easier and less costly to manufacture and purify than covalent Ab-SA conjugates.
Based on the advantageous properties of FPs, we have produced and characterized an anti-CD20 1F5 (scFv)4SA FP for PRIT of NHL. This FP maintains the full antigen and biotin binding capabilities of the parent 1F5 Ab and SA, and can be rapidly and quantitatively directed from the blood to the liver for hepatic processing by a biotinylated N-acetyl-galatosamine-containing CA. This attribute, coupled with the more rapid inherent clearance of the FP compared with chemical conjugates, results in extremely low serum concentrations of SA after CA administration. Our studies showed that both chemically conjugated 1F5 Ab-SA and anti-CD20 molecular FPs were markedly superior to the conventional radiolabeled Ab in directing radiotherapy to tumor cells. Biodistribution experiments demonstrated that the anti-CD20 1F5 (scFv)4SA molecular FP was superior to the chemically synthesized 1F5 Ab-SA covalent conjugate in this regard, as shown by improved tumor-to-normal organ ratios of absorbed radiation using the FP (Figure 4). The more efficient clearance of the FP construct from the bloodstream resulted in tumor-to-blood ratios, and thus tumor-to-normal organ ratios, of absorbed radioactivity that were at least 3- to 5-fold greater when the FP was employed compared with ratios obtained with the use of the pretargeted chemical conjugate.
Although the demonstration of substantial improvements in tumor-to-normal ratios of absorbed radiation using the 1F5 (scFv)4SA FP compared with the covalent conjugate were encouraging, it was important to perform therapy experiments comparing efficacies and toxicities using PRIT. These therapy experiments showed that optimal doses of the FP produced response and survival rates similar to those of the synthetic conjugate, though escalation of the dose of the FP was required to compensate for its more rapid clearance from the circulation. The anti-CD20 SA FP was safely delivered to mice using high doses of radiobiotin with minimal hematopoietic and nonhematopoietic toxicity. Myelosup-pression, particularly leukopenia, was the only significant toxicity observed and was dramatically less with all PRIT approaches than with directly labeled 90Y-DOTA-1F5 Ab (leukocyte nadir 3300 ± 1200 with (scFv)4-SA vs 800 ± 600 with 90Y-DOTA-1F5). Particular attention was paid to nephrotoxicity since the urogenital tissues may be particularly vulnerable due to renal excretion of 90Y-DOTA-biotin. Our results suggest that no acute or chronic radiation-induced renal or hepatic injury occurred as assessed by serial BUN, creatinine, and transaminase measurements performed for up to 12 months after the administration of radiolabeled biotin.
Although one prior report presents murine biodistribution data using an anti-CD20 (scFv)4 SA,25 we believe our report is the first to compare such an FP head-to-head with the corresponding Ab-SA chemical conjugate and with the corresponding directly labeled Ab. Furthermore, our study is the first to report controlled animal therapy experiments and toxicity data. Based on our findings, we conclude that anti-CD20 (scFv)4SA FPs produce in vivo biodistributions, toxicity profiles, and therapeutic results, when used with pretargeted 90Y-DOTA-biotin, that are markedly superior to directly 90Y-labeled anti-CD20 Ab and comparable to anti-CD20 Ab-SA chemical conjugates. These findings, coupled with the ability to generate a more homogeneous reagent with less lot-to-lot variability for clinical trials, lay the foundation for our ongoing efforts to produce 1F5 (scFv)4SA under cGMP conditions for human clinical trials in our Biological Production Facility.
Prepublished online as Blood First Edition Paper, March 23, 2006; DOI 10.1182/blood-2005-11-4327.
Supported by National Institutes of Health grants RO1 CA76 287 and K08 CA095 448, the Penny E. Petersen Memorial Fund, and a Specialized Center of Research (SCOR) grant from the Leukemia and Lymphoma Society of America (#7040). J.M.P. is supported by a Career Development Award from the Lymphoma Research Foundation and a Damon Runyon Career Development Award.
D.A. is employed by Aletheon Pharmaceuticals, whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.