Bone marrow normal lymphoid progenitors (CD19+, CD10+, and/or CD34+) are exquisitely sensitive to corticosteroids and other antileukemic drugs. We hypothesized that, in patients with B-lineage acute lymphoblastic leukemia (ALL), cells with this phenotype detected early in treatment should be leukemic rather than normal. We therefore developed a simple and inexpensive flow cytometric assay for such cells and prospectively applied it to bone marrow samples collected on day 19 from 380 children with B-lineage ALL. In 211 patients (55.5%), these cells represented 0.01% or more of the mononuclear cells; results correlated remarkably well with those of more complex flow cytometric and molecular minimal residual disease (MRD) evaluations. Among 84 uniformly treated children, the 10-year incidence of relapse or remission failure was 28.8% ± 7.1% (SE) for the 42 patients with 0.01% or more leukemic cells on day 19 detected by the simplified assay versus 4.8% ± 3.3% for the 42 patients with lower levels (P = .003). These assay results were the strongest predictor of outcome, even after adjustment for competing clinicobiologic variables. Thus, this new assay would enable most treatment centers to identify a high proportion of children with ALL who have an excellent early treatment response and a high likelihood of cure. (Blood. 2006;108:97-102)
Introduction
Response to treatment in childhood acute lymphoblastic leukemia (ALL) depends on numerous variables, including the clinicobiologic features of the disease, chemotherapy dosages and interactions, and the ability of individual patients to metabolize antileukemic drugs.1,2 One consistently useful prognostic indicator that reflects the collective impact of these variables is leukemia cytoreduction, defined as the rate of clearance of leukemic cells during remission induction chemotherapy. For example, levels of circulating lymphoblasts after 1 week of chemotherapy,3-7 or the detection of blast cells in the bone marrow by morphologic criteria after completion of remission induction therapy,8-10 are associated with an increased risk of relapse.
Despite their potential clinical usefulness, measurements of residual leukemia by morphologic analysis are inherently insensitive and imprecise,11,12 and they cannot be reliably used to identify patients whose leukemias are highly responsive to chemotherapy. Molecular and flow cytometric methods that allow detection of minimal residual disease (MRD) have a much higher (100-fold or more) sensitivity and precision than do assays based on morphology. Although the therapeutic benefits of treatment modifications based on results of MRD assays have not yet been established, these methods provide unique opportunities to pinpoint differences in initial treatment responsiveness,11,12 and to identify patients predicted to have superior outcome who might be candidates for trials testing less intensive therapies. One of the major obstacles to a wider use of MRD assays is their complexity, high costs, and requirement for considerable technical expertise. Hence, to extend the benefits of MRD detection to more children with ALL, we devised a relatively simple and inexpensive flow cytometric assay, based on expression of CD19, CD10, and CD34 antigens by bone marrow cells, that can reliably identify patients with profound leukemia cytoreduction on day 19 of remission induction therapy.
Patients, materials, and methods
Patients and treatment protocol
From August 1994 to November 2005, 386 patients with newly diagnosed B-lineage ALL were enrolled in Total Therapy studies at our institution and had residual disease studies by flow cytometry on day 19 of treatment (a morphologic assessment of treatment response was performed at this time, and surplus cells were used for residual disease studies). These studies were approved by the St Jude institutional review board, with informed consent obtained from the parents or guardians of each child in accordance with the Declaration of Helsinki. Diagnostic immunophenotyping and chromosomal and genetic analyses were performed by standard techniques.13-15
Initial treatment consisted of methotrexate and/or mercaptopurine,16 followed 4 days later by a 6-week remission induction therapy with prednisone, vincristine, daunorubicin, asparaginase, and etoposide plus cytarabine.17 Patients were considered to have lower-risk ALL if they were 1 to 9 years old with a presenting leukocyte count lower than 50 × 109/L or had a DNA index of 1.16 or more. All other patients were considered as higher risk, including those with the just-mentioned features and a CNS-3 status (ie, at least 5 leukocytes per microliter with identifiable blast cells in an atraumatic sample, or the presence of cranial nerve palsy); testicular leukemia; unfavorable cytogenetic abnormalities (t(9;22), t(4;11), t(1;19), MLL rearrangement, or near-haploidy); and/or 5% or more leukemic blasts in bone marrow on day 19 of remission induction.17 Upon induction of a complete clinical remission, all patients received 2 weeks of consolidation therapy with methotrexate and mercaptopurine, followed by risk-directed continuation therapy. For patients with higher-risk ALL, continuation therapy consisted of multiple drug pairs administered in weekly rotation, while patients with lower-risk leukemia received daily mercaptopurine and weekly methotrexate with prednisone plus vincristine pulse every 4 weeks. High-dose methotrexate was given every 8 weeks to all patients in the first year. Reinduction therapy (similar to that used initially) was administered from weeks 16 to 21. All patients received intrathecal therapy with methotrexate, hydrocortisone, and cytarabine for 1 year; cranial irradiation was reserved for those at very high risk of relapse (18 Gy) or those with central nervous system (CNS) status 3 at diagnosis (24 Gy).18 Patients deemed to have very high-risk leukemia underwent allogeneic hematopoietic stem cell transplantation while in clinical remission.
Assessment of treatment response with a simplified flow cytometry assay
Bone marrow aspirates were placed in preservative-free heparin and mononuclear cells separated on a density step (AccuPrep; Nycomed, Oslo, Norway). Mononuclear cells were labeled with anti-CD19 conjugated to peridinin chlorophyll protein (PerCP)-Cy5.5 or to allophycocyanin (APC), anti-CD10 conjugated to phycoerythrin (PE), and anti-CD34 conjugated to fluorescein isothiocyanate (FITC) or PerCP (all from BD Biosciences, San Jose, CA). After 10 minutes of incubation, the cells were washed twice with phosphate-buffered saline containing 0.5% bovine serum albumin and 0.5% sodium azide, and then fixed with 0.5% paraformaldehyde. Cell staining was analyzed with a FACScan or a FACSCalibur flow cytometer and Cell Quest software (Becton Dickinson, San Jose, CA). Between 1 × 105 and 5 × 105 mononuclear cells were analyzed in each sample. After gating on CD19+ cells with lymphoid morphology (as defined by their forward and side light-scattering properties), we determined the percentages of CD19+ cells expressing CD10 and/or CD34. Data were recorded without the observer's knowledge of a patient's clinical status or diagnostic features, except immunophenotype.
Conventional flow cytometric and molecular assessment of MRD
Flow cytometric studies of MRD, using 4-color analysis and multiple marker combinations individualized for each patient were performed as previously described.19,20 For each case, marker combinations allowing the identification of 1 leukemic cell per 104 normal nucleated bone marrow cells or greater were selected at diagnosis and then applied during clinical remission.
Polymerase chain reaction (PCR) studies of MRD were performed using immunoglobulin heavy chain and T-cell receptor gene rearrangements as targets, as previously described.21,22 Leukemia-associated rearrangements were identified at diagnosis, and primers complementary to the N-region nucleotides of the leukemic gene rearrangement were applied in each patient. MRD was quantified by either a limiting dilution method or a real-time PCR assay. In the former, leukemic cell DNA was serially diluted in 10-fold increments with DNA prepared from pooled peripheral-blood mononuclear cells; MRD was quantified with use of 10 replicates and Poisson statistics, as previously described.23,24 In the latter assay, diagnostic DNA was serially diluted with DNA prepared from normal peripheralblood mononuclear cells and standard curves were constructed; fluorescence data were collected during the annealing/extension phase of every cycle using the ABI PRISM 7700 Sequence Detection System containing a 96-well thermal cycler (PE Biosystems, Foster City, CA).
Statistical analysis
Differences in the distribution of clinicobiologic presenting features by level of CD19+ cells coexpressing CD10 and/or CD34 on day 19 were compared by the exact chi-square test and Fisher exact test. Correlations between the simplified flow cytometric assay and more complex MRD assays were assessed by the Spearman rank correlation test. For the cumulative incidence of induction failure and ALL relapse analysis, any leukemia relapse or failure to achieve remission was computed; death due to any cause was regarded as a competing event; there were no occurrences of second malignancies or myelodysplastic syndrome in the patient cohort analyzed for treatment outcome. Patients who achieved remission and were still alive without any event were censored at the time of last follow-up (median, 7.7 years from diagnosis). The risk of induction failure or ALL relapse and competing risks were tested with the method described by Gray25 ; the estimation was produced as described by Kalbfleisch and Prentice.26 Multiple regression modeling of subdistribution functions in competing risks, with adjustment for other prominent risk factors in childhoodALL, was used to further assess the association between residual disease on day 19 and the occurrence of leukemic relapse or induction failure.
Results
Identification of residual lymphoblasts by flow cytometry
In the bone marrow samples of healthy individuals, expression of CD19 with CD10 and/or CD34 denotes normal B-lymphoid precursors. These cells are highly sensitive to glucocorticoids, as demonstrated by their nearly complete eradication in patients receiving glucocorticoids for diseases other than leukemia (E.C.-S., D.C., unpublished data, March 1991). To determine whether remission induction therapy for ALL, which universally includes glucocorticoids such as prednisone or dexamethasone, depletes CD19+ cells expressing CD10 and/or CD34, we analyzed bone marrow samples collected from 11 patients with T-lineage ALL after 2 weeks of remission induction therapy (day 19). To identify normal B-cell precursors, we used flow cytometry and antibodies to these 3 markers. In all 11 patients, CD19+ cells expressing CD10 and/or CD34 were either undetectable or represented less than 0.01% of bone marrow mononuclear cells.
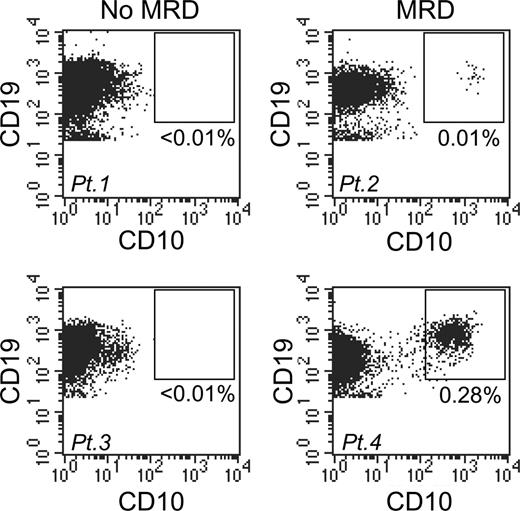
A simplified flow cytometric assay to measure early treatment response in childhood B-lineage ALL. Bone marrow samples from 4 patients were obtained on day 19 of remission induction therapy and stained with anti-CD19, anti-CD10, and anti-CD34 antibodies. Flow cytometric dot plots show CD19 and CD10 expression among mononuclear cells. In patients 1 and 3 (left panels), CD19+ coexpressing CD10 (and CD34; not shown) were undetectable, whereas in patients 2 and 4 (right panels) they constituted 0.01% and 0.28%, respectively, of mononuclear cells.
A simplified flow cytometric assay to measure early treatment response in childhood B-lineage ALL. Bone marrow samples from 4 patients were obtained on day 19 of remission induction therapy and stained with anti-CD19, anti-CD10, and anti-CD34 antibodies. Flow cytometric dot plots show CD19 and CD10 expression among mononuclear cells. In patients 1 and 3 (left panels), CD19+ coexpressing CD10 (and CD34; not shown) were undetectable, whereas in patients 2 and 4 (right panels) they constituted 0.01% and 0.28%, respectively, of mononuclear cells.
Among the 386 patients with B-lineage ALL studied at diagnosis, 380 (98.4%) had CD19+ leukemic cells that also expressed CD10 and/or CD34. Leukemic blasts from the remaining 6 patients lacked expression of CD34 and CD10, and therefore were excluded from further analysis. We then prospectively measured the percentage of CD19+ cells with CD10 and/or CD34 coexpression among bone marrow mononuclear cells collected on day 19 of remission induction chemotherapy from the 380 children whose leukemic cells had the requisite phenotype. In 211 patients (55.5%), 0.01% or more of these cells were CD19+ with coexpression of CD10 and/or CD34 (Figure 1). The levels of such cells were 0.01% to less than 0.1% in 76 patients, 0.1% to less than 1% in 65 patients, and 1% or more in 70 patients. In the remaining 169 patients (44.5%), cells expressing the base phenotype were below the standard limit of detection with our technique (0.01%; Figure 1).
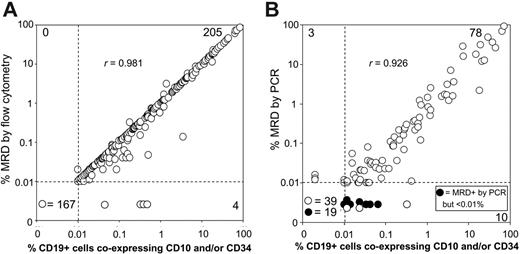
Relation between results of the simplified flow cytometric assays with those of more complex MRD assays. Bone marrow samples collected on day 19 of remission induction therapy from children with B-lineage ALL were examined for the presence of CD19+ cells coexpressing CD10 and/or CD34. Results were compared with those obtained with several sets of 4-antibody combinations in 376 patients (A), and with those of PCR amplification of immunoglobulin and T-cell receptor genes in 149 patients (B). Results of Spearman rank correlations are shown.
Relation between results of the simplified flow cytometric assays with those of more complex MRD assays. Bone marrow samples collected on day 19 of remission induction therapy from children with B-lineage ALL were examined for the presence of CD19+ cells coexpressing CD10 and/or CD34. Results were compared with those obtained with several sets of 4-antibody combinations in 376 patients (A), and with those of PCR amplification of immunoglobulin and T-cell receptor genes in 149 patients (B). Results of Spearman rank correlations are shown.
Of the 380 samples studied on day 19, 38 (10%) also had leukemic lymphoblasts clearly identifiable by morphology; in 28 samples, they represented 5% or more of the bone marrow mononuclear cells, while in 10 samples, they ranged from 1% to 4%. All samples morphologically positive for leukemic lymphoblasts had CD19+ cells coexpressing CD10 and/or CD34 by flow cytometry (median, 13.4%; range, 0.31%-85%). The remaining 342 samples (90%) lacked leukemic lymphoblasts recognizable by their morphology. However, 173 (50.6%) had CD19+ cells coexpressing CD10 and/or CD34 (median, 0.15%; range, 0.01%-29%). Nine of these samples had more than 5% CD19+ cells that also expressed CD10 and/or CD34. All contained more than 5% lymphoid cells by morphology, but these cells were deemed to be mature lymphocytes, not leukemic cells.
Correlation between levels of CD19+ lymphoblasts on day 19 and residual leukemia measured by standard MRD assays
To determine whether CD19+ cells with CD10 and/or CD34 coexpression, detected on day 19, represent residual leukemic cells or normal B-cell progenitors, we re-examined the samples for MRD using 2 established assays: (1) flow cytometric detection of aberrant immunophenotypes with different 4-color combinations selected according to the immunophenotype of leukemic cells at diagnosis and (2) PCR detection of immunoglobulin or T-cell receptor gene rearrangements.
In comparisons based on 376 patients (4 lacked leukemic cells with immunophenotypic features that differed from those of normal B-lymphoid progenitors), there was a remarkable concordance of results between the simplified assay and the more complex flow cytometric evaluation (Figure 2A). Inconsistent results were obtained in only 4 (1.1%) of the 376 samples, in which the simplified assay detected low levels of ostensibly leukemic lymphoid cells (median, 0.35%; range, 0.04%-047%), whereas the full MRD evaluation indicated that these were normal, not leukemic, B-cell progenitors.
Figure 2B compares results of the simplified assay with those of PCR amplification of antigen-receptor genes in 149 patients. With a threshold detection level of 0.01%, 3 samples were MRD negative by the simplified flow cytometric assay but MRD positive by PCR, although at very low levels (0.012%, 0.013%, and 0.016%). Ten additional samples had 0.01% or more lymphoblasts by the flow cytometric assay but had MRD levels less than 0.01% by PCR. Leukemia-associated PCR signals were detected in 7 of these samples, indicating MRD levels of 0.001% to less than 0.01%, while in the remaining 3, MRD was undetectable by PCR, suggesting that the cells could have been normal lymphoid progenitors.
Relation between levels of CD19+ lymphoblasts on day 19, clinicobiologic features of the disease, and treatment outcome
The presence or absence of residual CD19+ lymphoblasts coexpressing CD10 and/or CD34 on day 19 was not significantly related to age, sex, race, leukocyte count, presence of blasts in the CNS, or National Cancer Institute (NCI) risk status (Table 1). Among the biologic features examined, the rates of MRD detection by this simplified method did not differ significantly according to ploidy, presence of the t(4;11) and/or MLL gene rearrangements, or the t(1;19) and/or E2A-PBX1 transcripts. There was, however, a strong association between detection of CD19+ cells coexpressing CD10 and/or CD34 and the presence of Philadelphia (Ph) chromosome: all 13 cases with this unfavorable genetic abnormality27 had a positive assay finding (P = .001). Conversely, patients with TEL-AML1 fusion, a prognostically favorable feature,28 were significantly less likely to have residual disease (≥ 0.01%) than were patients lacking this abnormality (15% versus 77%, P < .001).
To determine the prognostic impact of detecting CD19+ cells with CD10 and/or CD34 on day 19, we analyzed the treatment outcome for 84 children enrolled, from August 1994 to February 1998, in a single program of chemotherapy (Total XIIIB), in which MRD status was not used for risk assignment. The 5-year cumulative incidence of relapse or failure to achieve clinical remission for patients with no immature CD19+ cells on day 19 (n = 42) was 4.8% ± 3.3%, compared with 23.8% ± 6.7% for the 42 patients with positive assay result findings, and 10-year estimates were 4.8% ± 3.3% versus 28.8% ± 7.1%, respectively (P = .003; Table 2; Figure 3). Notably, the 2 relapses in the group with negative findings occurred after the cessation of therapy, more than 4 years from diagnosis.
In a univariate analysis of recognized prognostic factors, including age, race, sex, presenting leukocyte counts, DNA index, and the presence of genetic abnormalities such as BCR-ABL, TEL-AML1, and MLL gene rearrangements, only the presence of the BCR-ABL abnormality was significantly associated with remission failure and ALL relapse (P = .004; Table 2). We next performed multiple regression analyses including presence or absence of CD19+ cells coexpressing CD10 and/or CD34 on day 19, BCR-ABL, as well as all other clinical and biologic parameters listed in Table 2. The presence of CD19+ cells coexpressing CD10 and/or CD34 on day 19 remained the only significant predictor of outcome (P = .025).
Prognostic significance of detecting CD19+ cells coexpressing CD10 and/or CD34 on day-19 bone marrow. Cumulative incidence of remission failure or ALL relapse according to the presence or absence (< 0.01%) of CD19+ cells coexpressing CD10 and/or CD34 in 84 children with B-lineage ALL enrolled in a single chemotherapy program.
Prognostic significance of detecting CD19+ cells coexpressing CD10 and/or CD34 on day-19 bone marrow. Cumulative incidence of remission failure or ALL relapse according to the presence or absence (< 0.01%) of CD19+ cells coexpressing CD10 and/or CD34 in 84 children with B-lineage ALL enrolled in a single chemotherapy program.
To determine if the simplified flow cytometric assay provided additional information to that provided by standard morphologic analysis, we focused our analysis on the 70 patients with negative morphology at day 19. Twenty-eight of the 70 patients had CD19+ cells coexpressing CD10 and/or CD34. These patients had significantly higher rates of remission failure or relapse at 5 years than the 42 patients with negative assay results (25.0% ± 8.4% versus 4.8% ± 3.3%; P = .012).
Rapid implementation of the new method in a resource-poor environment
To test the applicability of the simplified method in a resource-poor setting, we transferred it to a laboratory in Recife (Brazil) that lacked experience in MRD assays. Sample processing, antibody staining, and the initial flow cytometric analysis were performed exactly as specified by the protocol used in the St Jude laboratory, with approval of the local ethics committee. For quality control, flow cytometric data from all samples studied were transferred by compressing cytometer list-mode files, as previously described.29,30 More than 99% leukemic cells in the 38 patients with B-lineage ALL who were studied at diagnosis from November 2004 to December 2005 expressed the immature B-cell phenotype (CD19 with CD10 and/or CD34 coexpression). On day 19 of remission induction chemotherapy, CD19+ cells coexpressing CD10 and/or CD34 were detected in 26 (68%) of the 38 patients. Among the MRD-positive patients, levels of residual disease ranged from 0.01% to less than 0.1% in 8 patients, and from 0.1% to less than 1% in 6; they were 1% or more in 12 patients. Immature B cells were undetectable in samples from the remaining 12 patients. When repeated on day 26 in 20 patients, the test yielded negative results for 6 patients who had been MRD negative on day 19. Of the 14 patients who were MRD positive on day 19, 10 remained positive on day 26, while 4 had become MRD negative. Although based on limited sampling, these results suggest that the simplified assay can be effectively implemented to monitor MRD in laboratories with limited resources and no prior specific experience with MRD assays.
Discussion
Successful modern treatment of childhood ALL relies on the early assessment of the relapse hazard in individual patients.1,31 The initial response of leukemia cells to treatment has consistently been shown to be a reliable prognostic indicator,3-10 and its evaluation has been significantly enhanced by methods that allow detection of submicroscopic levels of leukemia, defined as minimal residual disease or MRD.19,32,33 Universal application of MRD assays would be highly desirable, but so far progress toward this has been slow because of the complexity and high costs of MRD evaluation. To address this problem, we developed a simple assay to detect immature B cells after 2 weeks of remission induction therapy, hypothesizing that MRD assessment at this interval would allow the identification of patients with an excellent early treatment response and a high probability of continued relapse-free survival.34,35 The rationale for this strategy was straightforward: normal immature CD19+ cells (ie, those expressing CD10 and/or CD34) are consistently undetectable in bone marrow samples collected from children with T-lineage ALL after 2 weeks of remission induction chemotherapy, because of their exquisite sensitivity to glucocorticoids and other antileukemic drugs. We therefore reasoned that any cells with this immunophenotype detected in patients with B-lineage ALL on day 19 of induction treatment would likely be residual leukemic cells. Our results demonstrate that very low levels (< 0.01%) of CD19+ cells coexpressing CD10 and/or CD34 during this stage of treatment identify a subgroup of patients (approximately 45%) with an excellent treatment outcome irrespective of other presenting clinical and biologic features.
Previous studies have shown that the morphologic detection of leukemic cells in day-15 bone marrow is prognostically important.8-10 However, morphologic assessment of bone marrow for residual leukemia has major disadvantages, notably the inability to consistently distinguish ALL cells from lymphocytes and undifferentiated hematopoietic cells. The assay we describe has the potential to overcome these limitations. In addition to the close correlation of its results with those of well-established MRD assays, we demonstrated the sensitivity of the simplified method, showing that 50% of so-called morphologically negative marrows in fact contained residual disease by the new assay. Most important, perhaps, patients in whom immature CD19+ cells were undetectable had remission-failure and long-term relapse hazards of less than 5%. In contrast to the sophisticated MRD assays now in use at some treatment research centers, the method presented here should be easy to implement in any center equipped with a basic single-laser flow cytometer. Moreover, the identification of immature CD19+ cells does not require extensive experience, and the assay is highly affordable (total cost of reagents for the evaluation of a single marrow is < 20 US dollars). The exportability of the assay to a resource-poor setting is illustrated by its successful transfer to a laboratory in Recife, Brazil, which supports a leukemia treatment program with improving results.30
Despite its ability to eradicate B-lineage ALL in all but the most resistant cases, intensive chemotherapy has proved to be a double-edged sword, resulting in serious and life-threatening sequelae.1 In developing countries, intensive chemotherapy might carry an unacceptably high risk of infections and treatment-related toxicities.36,37 The potential for overtreatment of childhood ALL patients is implied by the results achieved with previous, less intensive regimens (usually prednisone, vincristine, and daunorubicin or asparaginase for remission induction, followed by antimetabolite-based maintenance therapy) that consistently cured more than one third of patients with only minimal toxicity,38 and by results of a more recent clinical trial that yielded 5-year event-free survival rates of nearly 60% with only 1 year of treatment.39 Clearly, the identification of patients who could be cured with less toxic therapy is crucial to further progress in childhood ALL therapy, especially in terms of improvements in quality of life. Thus, on the strength of the study reported here, the Recife center has begun to test a new treatment strategy in which patients with no detectable CD19+ cells coexpressing CD10 and/or CD34 after 2 weeks of remission induction therapy are offered a regimen of reduced-intensity chemotherapy. Treatment-related mortality with standard ALL protocols at this center has been around 15%, and infection complications have been the most important problem.30 In the new protocol, reintensification of induction is omitted for B-lineage ALL patients with less than 0.01% CD19+ cells coexpressing CD10 and/or CD34 on day 19; these patients receive only one reinduction, and vincristine and prednisone pulses for the first year of maintenance only.
Lymphoblasts expressing CD19 with CD10 and/or CD34 are a typical finding in B-lineage ALL. Indeed, only 6 of the 386 patients studied at diagnosis lacked expression of both CD10 and CD34. Obviously, in these cases, one should not regard the lack of CD19+ cells coexpressing CD10 and/or CD34 during induction therapy as an indication of good treatment response. Rather, alternative strategies are needed to measure treatment response in such patients. One could, for example, combine anti-CD19 with anti-surface immunoglobulin (Ig) or anti-terminal deoxynucleotidyl transferase (TdT) antibodies to detect CD19+ surface Ig- or CD19+ TdT+ cells. Determining the cases in which alternative markers are needed to avoid false-negative results during disease monitoring should not be difficult, as CD19, CD10, and CD34 are almost universally used for diagnostic phenotyping in childhood ALL. Although we used Ficoll-separated mononuclear cells in our analyses, the same results could be achieved with samples in which red cells are lysed rather than removed by density gradient centrifugation. It may also be possible to apply the simplified flow cytometric assay near the end of remission induction therapy, without any loss of prognostic significance. However, use of the assay at other time points during or after treatment, when normal B-cell progenitors are most likely to be present, will inevitably yield false-positive results. At these time points, more complex flow cytometric and molecular MRD assays are required. Finally, our assay cannot be applied in cases of T-lineage ALL, for which a simple assay tracking cells expressing TdT+ CD3+ might be informative.
Children with B-lineage ALL account for nearly 85% of all pediatric cases of ALL. In principle, a substantial proportion of these patients would benefit from less intensive therapy that is specified by most contemporary protocols. For this strategy to succeed, it will be necessary to identify all patients whose leukemia is highly sensitive to remission induction therapy. We suggest that the simplified flow cytometric assay described here would provide the means to achieve this goal and could be readily applied in centers with only minimal laboratory resources.
Prepublished online as Blood First Edition Paper, March 14, 2006; DOI 10.1182/blood-2006-01-0066.
Supported by grants CA60419, CA5229, and CA21765 from the National Cancer Institute, and by the American Lebanese Syrian Associated Charities (ALSAC). C.-H.P. is an American Cancer Society-F. M. Kirby Clinical Research Professor.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Chris Clark, Laura Key, Peixin Liu, and Mo Mehrpooya for technical assistance; Teresa Santiago and Veruska Alves for studies in Recife; Cheng Cheng and Michael Hancock for guidance in the statistical analysis; and John Gilbert for critical review of the paper.