Abstract
We evaluated the role of rituximab (R) both in remission induction and maintenance treatment of relapsed/resistant follicular lymphoma (FL). A total of 465 patients were randomized to induction with 6 cycles of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) (every 3 weeks) or R-CHOP (R: 375 mg/m2 intravenously, day 1). Those in complete remission (CR) or partial remission (PR) were randomized to maintenance with R (375 mg/m2 intravenously once every 3 months for a maximum of 2 years) or observation. R-CHOP induction yielded an increased overall response rate (CHOP, 72.3%; R-CHOP, 85.1%; P < .001) and CR rate (CHOP, 15.6%; R-CHOP, 29.5%; P < .001). Median progression-free survival (PFS) from first randomization was 20.2 months after CHOP versus 33.1 months after R-CHOP (hazard ratio [HR], 0.65; P < .001). Rituximab maintenance yielded a median PFS from second randomization of 51.5 months versus 14.9 months with observation (HR, 0.40; P < .001). Improved PFS was found both after induction with CHOP (HR, 0.30; P < .001) and R-CHOP (HR, 0.54; P = .004). R maintenance also improved overall survival from second randomization: 85% at 3 years versus 77% with observation (HR, 0.52; P = .011). This is the first trial showing that in relapsed/resistant FL rituximab maintenance considerably improves PFS not only after CHOP but also after R-CHOP induction.
Introduction
For patients with follicular lymphoma (FL) chemotherapy alone has not resulted in improved overall survival (OS) over the past 30 years.1-4 Although in most patients complete remissions (CRs) or partial remissions (PRs) can be obtained with either single agents or combination chemotherapy, the clinical course is characterized by a high relapse rate. After relapse, both the response rate and relapse-free survival after subsequent salvage treatment regimens steadily decrease, resulting in a median survival of only 4 to 5 years after first relapse.5-8 Therefore, new treatment modalities resulting in increased progression-free survival (PFS) and OS are urgently required. Optimal treatment of patients relapsed after 1 or 2 chemotherapy regimens is largely unknown.
Rituximab (R) is a chimeric murine/human anti-CD20 monoclonal antibody capable of killing CD20+ lymphoma cells. Effector mechanisms include complement-mediated cytotoxicity, antibody-dependent cellular cytotoxicity, and possibly direct induction of apoptosis.9,10 In the nonrandomized pivotal study in 166 relapsed low-grade lymphoma patients, monotherapy with rituximab resulted in a response rate of 48%, with a 6% complete remission (CR) rate and a median time to progression in responding patients of 13 months.11 Toxicity was generally mild to moderate (grade 1 or 2) and occurred primarily with the first infusion.11 In a subsequent small phase 2 study, the combination of R with cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) was shown to be safe and effective.12
Treatment results in FL might not only be improved by more effective induction regimens, but also by maintenance treatment defined as continued treatment beyond induction therapy. Maintenance treatment with cytotoxic agents has been shown to improve PFS but not OS.13-16 This prolongation of PFS was achieved at the costs of increased toxicity, reduced patient well-being, and increased risk of secondary malignancies. In a recent meta-analysis, interferon maintenance treatment showed a survival benefit in FL when given in conjunction with intensive chemotherapy and at certain dose levels. However, the benefit of maintenance was not consistent across all studies, and toxicity was considerable.17 Because of its efficacy as monotherapy and its favorable pharmacokinetic and safety profile, maintenance treatment with rituximab might be both effective and well-tolerated.
In view of (1) the efficacy of rituximab monotherapy in relapsed low-grade lymphoma,11 (2) the feasibility of combining rituximab with cytotoxic drugs,12 and (3) the theoretical potential of such combinations to clear minimal residual disease, we decided in 1998 to launch a phase 3 randomized clinical trial in patients with relapsed or resistant FL with 2 main objectives: first, to compare response rates with CHOP and R-CHOP and, second, to establish the effect of maintenance treatment with rituximab on progression-free survival (PFS).
Patients, materials, and methods
Patients
This randomized (1:1) open-label phase 3 intergroup study was conducted at 130 centers in Canada, Australia/New Zealand, Europe, and South Africa from November 1998 to April 2004. Patients eligible for the study were older than 18 years of age with a CD20+ grade 1 to 3 FL, Ann Arbor stage III or IV at initial diagnosis, and relapse after or resistance to a maximum of 2 nonanthracycline-containing systemic chemotherapy regimens. A previous regimen was defined as at least 2 months of single-agent therapy (eg, chlorambucil) and/or at least 2 consecutive cycles of polychemotherapy (eg, cyclophosphamide, vincristine, and prednisolone [CVP]) or purine analogs. Patients had to have at least 1 bidimensional measurable mass by either clinical or radiologic examination. World Health Organization (WHO) performance status had to be 2 or below. Major exclusion criteria were prior treatment with anthracyclines, rituximab, or autologous or allogeneic stem cell transplantation; more than 10 × 109/L circulating tumor cells; histologic transformation; known HIV positivity; symptomatic central nervous system (CNS) lymphoma; IgG levels below 3 g/L; and severe concomitant disease. Patient information and written informed consent were obtained according to the rules of the respective country and institute. The study was conducted according to the Declaration of Helsinki and the Guidelines for Good Clinical Practice.
Study design and treatment
Eligible patients were randomized to remission induction with either 6 cycles of standard CHOP (cyclophosphamide 750 mg/m2 intravenously, day 1; doxorubicin 50 mg/m2 intravenously, day 1; vincristine 1.4 mg/m2 intravenously, [maximum 2 mg], day 1; and prednisone 100 mg/d orally, days 1 to 5; once every 3 weeks) or CHOP plus rituximab (R) (375 mg/m2 intravenously at day 1 of each cycle of CHOP). After 3 cycles of CHOP with or without R, patients were evaluated for response. Those with stable disease or progression went off study. Responders received another 3 cycles of the assigned treatment. Patients with a CR or a PR after 6 cycles of therapy underwent a second randomization to either no further treatment (observation) or maintenance treatment with rituximab (375 mg/m2 intravenously once every 3 months until relapse or for a maximum of 2 years). Exclusion criteria for second randomization were no CR or PR upon induction treatment, IgG levels below 3 g/L, and active infection.
First randomization was stratified by center, previous treatment with purine analogs, age, number of previous induction treatments and best response previously obtained (CR/PR/no change [NC]/progressive disease [PD]), time since diagnosis (more than 2 years or 2 years or less), and bulky disease (more than 10 cm or 10 cm or less) using a minimization procedure. The second randomization was stratified according to the treatment allocated by the first randomization, the quality of the response obtained after induction (CR/PR), and center.
Responses after induction treatment were evaluated by physical examination, hematology and chemistry, computed tomography (CT) scans (obligatory), and bone marrow biopsies (when indicated) and assessed according to the Lymphoma Expert's Confirmation of Response (LEXCOR) criteria.18 Patients lacking bone marrow evaluation but without evidence of disease on physical examination and CT scans were scored as partial remissions.
During the 2 years of R maintenance/observation, physical examination and hematology and chemistry were performed at least every 3 months and thereafter once every 4 to 6 months. In this large multicenter international study it was decided to adhere as much as possible to the daily practice in the participating countries. Thus, during maintenance/observation CT and bone marrow examinations were performed only on indication. A central pathology review was performed by all participating groups.
The trial was designed to detect a 10% difference (from 70% to 80%) in the overall response rate to induction chemotherapy and had to recruit 600 patients (alpha = 0.05, beta = 0.22, 2-sided test). The final analysis of maintenance was foreseen after 201 progressions or deaths to detect a 14% difference in the 2-year PFS (from 40% to 54%; alpha = 0.05, beta = 0.2, 2-sided test). An interim analysis of safety was planned after inclusion of 50 patients and 2 interim analyses of efficacy after inclusion of 200 and 400 patients (Haybittle and Peto strategy, P < .001).
Statistical analysis
All primary analyses were conducted following the intention to treat (ITT) principle. The primary end point for the induction phase was the response to treatment. Secondary end points were PFS and OS from first randomization. Response rates were compared using the Mantel-Haenszel test for trend on 4 ordered categories (CR/PR/NC/PD). For the primary analysis, nonassessable patients were excluded. For sensitivity analyses, nonassessable cases were considered as progressions. The primary end point for the maintenance phase was PFS (defined as interval between the date of second randomization and date of first relapse, progression, or death), and the secondary end point was OS from second randomization. The principal analysis of PFS and OS was done with the log-rank test and sensitivity analyses done with Cox regression analysis with adjustment for type of induction treatment and response. Kaplan-Meier curves were calculated to graphically show the differences between the treatment arms. All P values given are 2-sided.
In February 2004, a preplanned second interim analysis of the present study was reviewed by the Independent Data Monitoring Committee (IDMC) of this study. At that time, 461 patients had been included (369 evaluable for response) and 319 patients had been randomized for maintenance treatment (268 evaluable). The results revealed that the primary end points for both the induction and maintenance part of the study had been reached, and the formal criteria for stopping the trial had been met. Subgroup analysis as requested by the IDMC confirmed the benefit of R maintenance in the CHOP subgroup but not yet in the R-CHOP subgroup. It was therefore suggested to amend the protocol with all patients receiving R-CHOP for induction treatment followed by randomization to R maintenance therapy or no further treatment. Hence, recruitment to the trial was suspended in April 2004 during preparation of a major protocol amendment. In the meantime, all data were retrospectively monitored on site, and all pending queries were solved to perform a final analysis including all patients recruited to the study by April 2004. Because the study was conducted at 130 sites and by 8 clinical study groups, the monitoring and data cleaning process was only completed in September 2005. Thus, an updated data set with additional 19 months of median follow-up was available for the final analysis. After reviewing the data of this final analysis, the IDMC recommended not to reopen the trial because the primary question of the amended protocol had already been answered.
Results
Patients
A total of 474 patients with relapsed/resistant FL were randomly assigned to receive induction treatment with CHOP or R-CHOP. Nine patients had to be excluded because of missing informed consent forms. Therefore, all analyses are restricted to 465 patients (231 CHOP and 234 R-CHOP). Recruitment was stopped after the preplanned second interim analysis for efficacy because the criteria for early discontinuation were met both for induction and maintenance. Baseline demographics and other characteristics of the 2 groups were well balanced (Table 1). Because the Follicular Lymphoma International Prognostic Index (FLIPI) was only published in 2004,19 the FLIPI score was assessed retrospectively for our patients and thus was not used as a stratification factor. However, both study arms were well balanced, with 70% of the patients having intermediate (FLIPI score 2) or high-risk (FLIPI score 3 or higher) disease at study entry (Table 1). According to local pathology, 98% of the patients had FL. Central pathology data are available for 82% of all patients. The overall concordance rate between local and central assessment for all subtypes of FL was 93% in both treatment arms.
In both groups, about 80% had received only one prior treatment, almost equally consisting of single-agent therapy or polychemotherapy. In both arms, only 9% of the patients had been treated previously with purine analogs. Best response to prior treatment was similar in both study arms. A total of 17% and 16% of patients were resistant to their prior treatment in the CHOP and R-CHOP arms, respectively (Table 1). Three randomized patients never started protocol treatment—1 because of rapid progression and 2 refusals. The 6 cycles of protocol therapy could be completed in 81% of the patients in the CHOP arm and in 89% in the R-CHOP arm. Dose density for doxorubicin and cyclophosphamide was similar in both arms. Most protocol discontinuations occurred at the time of the first response evaluation, after the third cycle of treatment.
The 334 patients randomized to the maintenance phase were well balanced for baseline characteristics at study entry, FLIPI score (2 or higher in 70% and 66% in the observation and maintenance arms, respectively), type of induction treatment received, and response to induction (in both arms, 29% CR and 71% PR). In both arms, more patients had received R-CHOP during induction (59% in the observation arm and 55% in the maintenance arm), reflecting the higher efficacy of R-CHOP as compared with CHOP in terms of response induction. Maintenance treatment was started a median of 7 weeks (range, 3 to 16 weeks) after the end of the last induction cycle.
Efficacy: induction phase
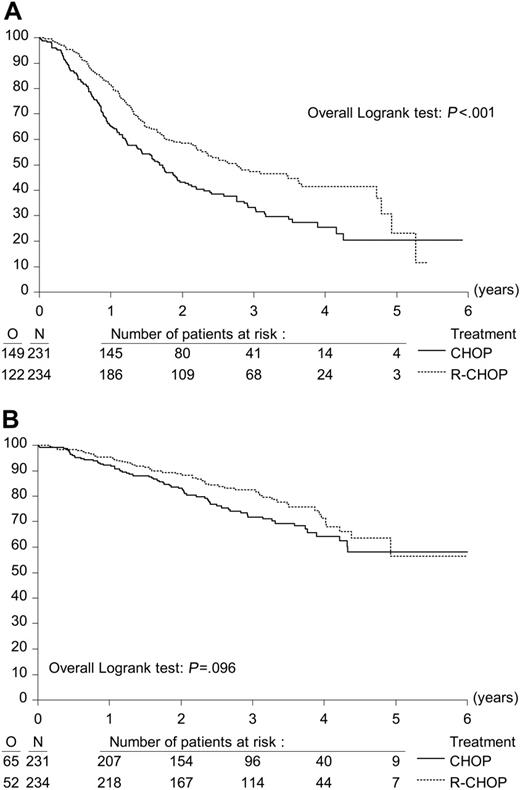
The addition of rituximab significantly increased both overall response and complete remission rates. Overall response rates were 72.3% and 85.1% after CHOP and R-CHOP induction treatment, respectively (P < .001). The CR rate was 15.6% in patients receiving CHOP and 29.5% in patients treated with R-CHOP (P < .001) (Table 2). The partial response rate was 56.7% in the CHOP arm and 55.6% in the R-CHOP arm (nonsignificant [NS]). With a median follow-up from first randomization of 39.4 months, median PFS from first randomization was 20.2 months in the CHOP group versus 33.1 months in the R-CHOP group (P < .001, log-rank test; Figure 1A). Hazard ratio (HR) for the R-CHOP group was 0.65. OS at 3 years from first randomization was 71.9% in the CHOP arm and 82.5% in the R-CHOP arm (P = .096, log-rank test; HR, 0.74; Figure 1B).
Efficacy: maintenance phase
Of the 366 patients having responded to induction treatment (with either CHOP or R-CHOP) 32 were not randomized for maintenance treatment—17 because of low IG levels (9 in the CHOP arm, 8 in R-CHOP); 8 patients because they were still on CHOP induction when the trial was put on hold because of the results of the first interim analysis (these patients received R maintenance treatment on a compassionate need basis; they were included in the analysis of response to induction but were excluded from the analysis of maintenance treatment); 1 because of a secondary neoplasia; and 1 because of active infection. There were 3 refusals and 2 ineligibilities due to administrative problems.
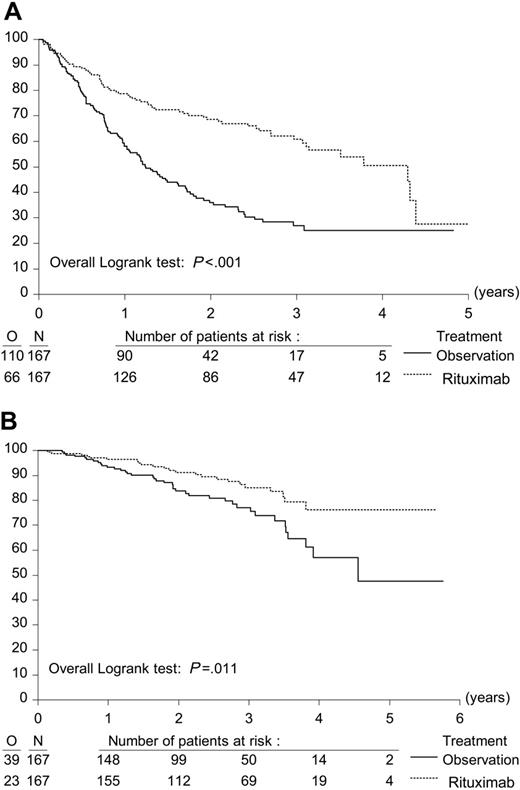
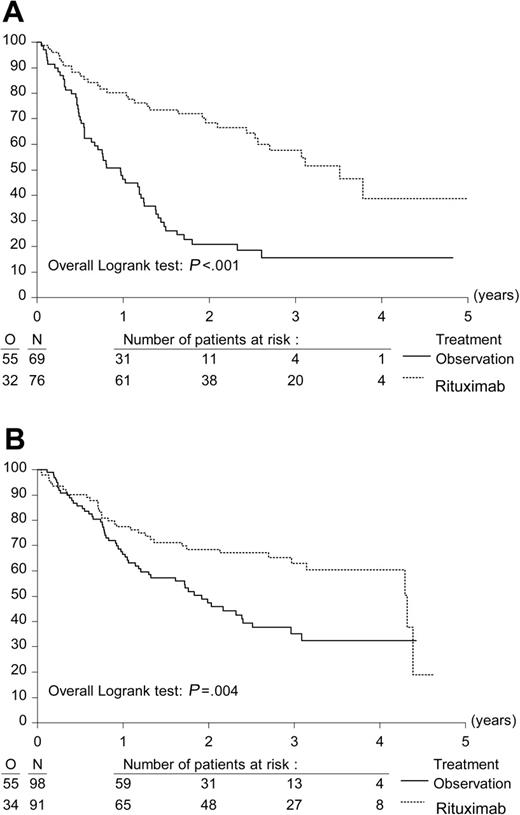
A total of 334 eligible patients were randomly assigned to R maintenance treatment (n = 167) for 2 years or observation (n = 167). In each study arm, 1 patient did not start allocated treatment because of progression immediately after randomization. At the time of last follow-up, 41 patients were still under maintenance treatment or observation. With a median follow-up from second randomization of 33.3 months, median PFS from second randomization was 51.5 months in the R maintenance arm versus 14.9 months in the observation arm (P < .001, log-rank test). The hazard ratio for R maintenance treatment compared with observation was 0.40; P < .001 (Figure 2A). Because the difference in PFS was highly significant, a further analysis was carried out to evaluate whether the benefits of maintenance applied to patients treated both with CHOP and R-CHOP. After CHOP induction, R maintenance resulted in a median PFS from second randomization of 42.2 months versus 11.6 months in the observation arm (HR, 0.30; P < .001). After R-CHOP induction, the figures were 51.8 months and 23.0 months, respectively (HR, 0.54; P = .004) (Figure 3). Similarly, R maintenance resulted in a highly significant increase in PFS both in patients who had a PR after induction treatment and those who had obtained a CR (data not shown).
Effect of addition of R (rituximab) to CHOP remission induction on progression-free survival and overall survival. Kaplan-Meier plots of progression-free survival and overall survival from first randomization. (A) Progression-free survival after CHOP (n = 231) and R-CHOP (n = 234) remission induction treatment. (B) Overall survival after CHOP (n = 231) and R-CHOP (n = 234) remission induction treatment.
Effect of addition of R (rituximab) to CHOP remission induction on progression-free survival and overall survival. Kaplan-Meier plots of progression-free survival and overall survival from first randomization. (A) Progression-free survival after CHOP (n = 231) and R-CHOP (n = 234) remission induction treatment. (B) Overall survival after CHOP (n = 231) and R-CHOP (n = 234) remission induction treatment.
R maintenance treatment increased 3-year overall survival rates (from second randomization) from 77.1% in the observation group to 85.1% in the R maintenance group (P = .011, log-rank test; Figure 2B). The hazard ratio for R maintenance compared with observation is 0.52. All sensitivity analyses confirmed the results of the principal analyses.
Safety
Induction. Grade 3-4 neutropenia was the most frequent adverse event (AE): 48.2% grade 3-4 in the CHOP arm and 54.7% in the R-CHOP arm (NS). More patients on R-CHOP experienced grade 3-4 allergy (CHOP, 0 patients; R-CHOP, 8) and skin reactions (CHOP, 17 patients; R-CHOP, 31). Six patients in the CHOP arm and 8 patients in the R-CHOP arm withdrew from treatment because of toxicity. Treatment-related mortality occurred in 2 patients in the CHOP group (1 sepsis, 1 respiratory distress syndrome) and in 1 patient in the R-CHOP group (pneumonia). During induction, hypogammaglobulinemia developed in about 5% of the patients. Indeed, 17 of the 366 responders to induction treatment were not eligible for second randomization because of IgG levels below the predefined threshold of 3 g/L. However, we did not find a correlation between the incidence of bacterial infections and decreased IgG levels.
Maintenance. During R maintenance treatment, neutropenia was the only significant AE: 10.8% in the R maintenance arm versus 5.4% in the observation arm (NS; P = 0.07, χ2). This probably contributed to the increased grade 3-4 infection rate: 9% in the maintenance group and 2.4% (P = .009, χ2) during observation, with most of these in the ear-nose-throat area. During maintenance 6 patients with therapy-related grade 3-4 adverse events (infection) were hospitalized. They fully recovered.
Only 6 of the 167 patients withdrew from R maintenance treatment because of toxicity (4 of the 6 due to infections). According to protocol, IgG (but not IgA and IgM) levels were measured every 3 months during maintenance treatment/observation. At second randomization the median IgG levels were just below the normal range in both arms (6.6 g/L in the observation arm and 6.5 g/L in the maintenance arm). Whereas during 2 years of observation the median IgG levels increased to within the normal range (7.3 g/L), IgG levels remained stable in the maintenance arm (6.3 g/L). Maintenance dose was delayed in only 1 patient and omitted in 2 patients at least once, because of low (below 3 g/L) IgG levels. No patient had to be withdrawn from R maintenance treatment due to persisting IgG levels below 3 g/L. There were no deaths related to R maintenance treatment.
Effect of R (rituximab) maintenance treatment on progression-free survival and overall survival. Kaplan-Meier plots of progression-free survival and overall survival from second randomization. (A) Progression-free survival after R maintenance therapy (n = 167) and observation (n = 167). (B) Overall survival after R maintenance therapy (n = 167) and observation (n = 167).
Effect of R (rituximab) maintenance treatment on progression-free survival and overall survival. Kaplan-Meier plots of progression-free survival and overall survival from second randomization. (A) Progression-free survival after R maintenance therapy (n = 167) and observation (n = 167). (B) Overall survival after R maintenance therapy (n = 167) and observation (n = 167).
Effect of R (rituximab) maintenance treatment on progression-free survival after remission induction with either CHOP or R-CHOP. Kaplan-Meier plots of progression-free survival from second randomization. (A) Progression-free survival after CHOP remission induction (n = 145). (B) Progression-free survival after R-CHOP remission induction (n = 189).
Effect of R (rituximab) maintenance treatment on progression-free survival after remission induction with either CHOP or R-CHOP. Kaplan-Meier plots of progression-free survival from second randomization. (A) Progression-free survival after CHOP remission induction (n = 145). (B) Progression-free survival after R-CHOP remission induction (n = 189).
Discussion
The final analysis of the European Organisation for Research and Treatment of Cancer (EORTC) 20981 Intergroup study has shown several important findings. Firstly, in patients with relapsed/resistant FL, remission induction with R-CHOP results in a highly significant increase in CR rate as compared with CHOP; secondly, R maintenance treatment significantly improves PFS and OS in patients responding to induction treatment; thirdly, R maintenance treatment achieves a considerable increase in PFS not only after remission induction with chemotherapy (CHOP) but also after immunochemotherapy (R-CHOP).
Since the start of the trial in late 1998, a considerable amount of data on efficacy and safety of rituximab in combination with different chemotherapy regimens as induction therapy for both previously untreated and pretreated patients has been published.20,21 There is a strong rationale for this combination because cytotoxic drugs and rituximab both have proven efficacy but different mechanisms of action and nonoverlapping toxicities. In addition, in vitro data have shown that rituximab may increase sensitivity of lymphoma cells to cytotoxic agents.9
In indolent lymphoma, remission induction with the combination of rituximab and chemotherapy has been shown to be superior to chemotherapy alone in several randomized phase 3 trials, both in previously untreated as well as in relapsed patients. In previously untreated patients, the addition of R to chemotherapy results in significantly better overall response and complete remission rates and improved PFS22-25 and OS.22,24 Our finding of a superior CR rate after R-CHOP in relapsed FL patients is in line with the results of the German Low Grade Lymphoma Study Group, which showed in a mixed group of relapsed/refractory indolent non-Hodgkin lymphoma (NHL) and mantle cell lymphoma patients that R-FCM (rituximab–fludarabine, cyclophosphamide, and mitoxantrone) yields significantly higher ORR and CR rates and prolongs PFS and OS when compared with FCM alone.26 In all these studies, addition of rituximab to chemotherapy did not result in increased toxicity.
In the past decades, maintenance therapy over a period of 12 to 24 months after induction treatment was evaluated using cytotoxic agents such as chlorambucil or cyclophosphamide13-16 or interferon-α.17,27-32 However, no consistent long-term benefit in terms of OS could be demonstrated, and prolonged administration of both chemotherapy and interferon-α are associated with significant toxicity and patient inconvenience.
Two randomized trials have investigated the efficacy of induction therapy with single-agent rituximab followed by rituximab maintenance treatment. Hainsworth et al randomized patients with relapsed or refractory indolent NHL to R maintenance or R retreatment at disease progression and found an approximate 4-fold increase in PFS for the former (31.1 months versus 7.4 months).33 However, the rituximab benefit (defined as date of study entry to date of next lymphoma treatment) was similar in both groups (31.3 versus 27.4 months, respectively). Because this was not part of our study, we do not have systematic information on the retreatment of patients who relapsed after either R maintenance or observation. However, because rituximab was registered and available in all participating countries, it has to be assumed that many patients will have received a rituximab-containing regimen, notably those who received rituximab neither during induction nor maintenance. Indeed, a preliminary analysis showed that in the patients in the observation arm, first post-protocol treatment (n = 85) was R monotherapy in 29% and R-chemo in 11%, versus 11% and 5%, respectively, in patients in the maintenance arm requiring post-protocol treatment (n = 56). The Swiss Group for Clinical Cancer Research (SAKK) 35-98 study showed an almost 2-fold increase in median event-free survival (EFS) by R maintenance in patients with untreated and relapsed FL (from 12 to 23 months).34
The efficacy of R maintenance therapy has also been investigated following treatment with different chemotherapy regimens. In previously untreated patients with indolent lymphoma (Eastern Cooperative Oncology Group [ECOG] 1496), R maintenance treatment after remission induction with CVP increased PFS by almost 3 years and improved OS in patients with high tumor burden.35 In all these studies, R maintenance treatment was well tolerated and did not lead to significantly higher rates of neutropenia, thrombocytopenia, and/or infection as compared with observation.
Our study is the first large randomized trial to show that in patients with relapsed/resistant FL, R maintenance treatment achieves a statistically highly significant and clinically very relevant improvement in PFS after induction treatment with chemotherapy plus rituximab. For the pooled CHOP and R-CHOP patients, R maintenance also improved OS: 85% at 3 years versus 77% with observation (HR, 0.52; P = .011). Of course, follow-up is still rather short for patients with FL, and longer follow-up is required to know whether the survival benefit will stand. Recently, a preliminary report of a randomized study by Hiddemann et al in a mixed population of relapsed FL and MCL also showed a significant improvement in response duration for patients receiving R maintenance after induction therapy with R-FCM (n = 119).36 In relapsed FL there has only been one randomized trial comparing chemotherapy and autologous stem cell transplantation.37 Although the number of patients was small and not balanced as to prognostic factors between the study arms, a clear benefit for autologous stem cell transplantation as to PFS and OS was shown. In view of the excellent PFS obtained with R-CHOP induction followed by R maintenance, future trials in relapsed FL should compare this (or a comparable) regimen with an optimal transplantation approach: R-chemotherapy induction and R-myeloablative treatment, and R maintenance after transplantation. This probably also applies to future trials in FL of autologous transplantation in first remission.
In conclusion, we have shown that R maintenance treatment results in a major improvement in PFS both after chemotherapy and immunochemotherapy and, most importantly, also in a better OS. Questions still to be answered relate to the optimal schedule (eg, single infusions every 2 to 3 months or 4 weekly infusions every 6 months) and duration of R maintenance (eg, 2 years or until progression), and whether the results of R-chemotherapy induction and R maintenance in relapsed/resistant FL will be similar in patients who have already received prior rituximab-containing regimens.
Appendix
The following investigators (listed in alphabetical order) included patients in the study.
National Cancer Institute of Canada (NCIC)
A. Belch, Cross Cancer Institute, Edmonton; G. Cantin, Hopital Du Saint Sacrement, Quebec; H. I. Chalchal, Allan Blair Cancer Centre, Regina; M. Crump, University Health Network–Oci/Princess Margaret Hospital, Toronto; P. Desjardins, Hopital Charles Lemoyne, Greenfield Park, Quebec; B. Findlay, Hotel Dieu Hospital, St Catharines; K. Grewal, Dr H. Bliss Murphy Cancer Centre, St John's; K. Howson-Jan, London Regional Cancer Center, London; K. Imrie, Toronto-Sunnybrook Regional Cancer Centre, Toronto; R. Klasa, BC Cancer Agency, Vancouver Cancer Centre, Vancouver; M. Kovacs, London Regional Cancer Center, London; B. Lesperance, Hopital Du Sacre-Coeur De Montreal, Montreal; J. Long, Waikato Hospital, Hamilton; P. Lopez, Northeastern Ontario Regional Cancer Center, Sudbury; J. Mathews, Windsor Regional Cancer Centre, Windsor; J. Matthews, Kingston Regional Cancer Center, Kingston; R. Meyer, Hamilton Health Sciences, Juravinski Cancer Centre, Hamilton; K. Murphy, BC Cancer Agency, Fraser Valley Cancer Centre, Surrey; B. Pressnail, Royal Victoria Hospital, Barrie; S. Robinson, Nova Scotia Cancer Centre, Halifax; S. Rubin, The Moncton Hospital, Moncton; L. Rudinskas, Humber River Regional Hospital, Toronto; C. Shustik, McGill University, Department of Oncology, Montreal; D. Soulieres, Hopital Notre-Dame Du Chum, Montreal; D. Stewart, Tom Baker Cancer Centre, Calgary; J. Sutherland, BC Cancer Agency, Centre for the Southern Interior, Kelowna.
British National Lymphoma Investigation (BNLI)
D. Bareford, City Hospital, Birmingham; K. Benstead, Cheltenham General Hospital, Cheltenham; P. C. Bevan, St Richard's Hospital, Chichester; N. Blessing, The Great Western Hospital, Swindon; A. K. Burnett, University of Wales College of Medicine, Cardiff; A. O'Callaghan, St Mary's Hospital, Portsmouth; R. Chasty, North Staffordshire Hospital, Stoke on Trent; J. Cullis, Salisbury District Hospital, Salisbury; D. Cunningham, Royal Marsden Hospital, Sutton; D. Dunlop, Royal Infirmary, University of Glasgow, Glasgow; M. S. Dyer, Leicester Royal Infirmary, Leicester; B. Hancock, Weston Park Hospital, Sheffield; C. Hatton, John Radcliffe Hospital, Oxford; A. M. O'Hea, Stoke Mandeville Hospital, Aylesbury; P. J. Hoskin, Mount Vernon Hospital, Northwood; Al-Ismail, Singelton Hospital, Swansea; E. Lee, Countess of Chester Hospital, Chester; M. Lyttelton, Kettering General Hospital, Kettering; M. Mackie, Western General Hospital, Edinburgh; R. Marcus, Addenbrooke's Hospital, Cambridge; E. Marshall, Clatterbridge Hospital, Bebington; T. Maughan, Velindre Hospital, Cardiff; G. J. Morgan, Leeds General Infirmary, Leeds; A. Morrison, Southern General Hospital, Glasgow; T. C. M. Morris, Belfast City Hospital, Belfast; J. T. Neilson, Russels Hall Hospital, Dudley; D. H. Parry, North West Wales National Health Service (NHS) Trust–Bangor Hospital, Gwynedd; R. Patmore, Hull Royal Infirmary, Hull; R. Pettengell, St Georges Hospital, London; A. Pettit, Royal Liverpool University Hospital, Liverpool; S. J. Proctor, Royal Victoria Infirmary, Newcastle-Upon-Tyne; A. J. Rathmell, The James Cook University Hospital, Cleveland; G. Satchi, Whiston Hospital, Prescot; P. J. Stableforth, Sandwell District General Hospital, Rhyl Denbighshire; A. Vranovsky, National Cancer Institute, Bratislava, Slovakia; J. Wimperis, Norfolk and Norwich Hospital, Norwich.
Hemato-Oncologie voor Volwassenen Nederland (HOVON)
J. W. Baars, The Netherlands Cancer Institute–Antoni van Leeuwenhoek Ziekenhuis, Amsterdam; H. Dankbaar, Stichting Streekziekenhuis, Hengelo; W. A. van Deijk, Rode Kruis Ziekenhuis, The Hague; A. C. Dullemond-Westland, Van Weel en Bethesda Ziekenhuis, Dirksland; A. A. van Houten, Zuiderziekenhuis, Rotterdam; P. Huijgens, Vrije Universiteit Medisch Centrum, Amsterdam; G. W. van Imhoff, Academisch Ziekenhuis Groningen, Groningen; J. Th. P. Janssen, Ziekenhuis Franciscus, Roosendaal; G. K. S. Jie, Atrium Medisch Centrum, Heerlen; F. H. W. Kauw, Albert Schweitzerziekenhuis-Sliedrecht, Sliedrecht; M. H. H. Kramer, Meander Medisch Centrum–De Lichtenberg, Amersfoort; M. B. L. Leys, St Clara Ziekenhuis, Rotterdam; G S. Liem, Laurentius Ziekenhuis Roermond, Roermond; H. M. Muller, Streekziekenhuizen Gooi-Noord, Blaricum; E. F. M. Posthuma, Reinier De Graaf Gasthuis, Delft; H. Th. J. Roerdink, Twee Steden Ziekenhuis–Locatie Tilburg, Tilburg; W. M. Smit, Medisch Spectrum Twente, Enschede; P. Sonneveld, Erasmus MC, Rotterdam; C. A. M. de Swart, Spaarne Ziekenhuis Haarlem, Haarlem; P. Vandenberghe, U. Z. Gasthuisberg, Leuven; M. B. van't Veer, Erasmus University Medical Center, Rotterdam; J. J. Wegman, Deventer Ziekenhuizen, Deventer.
Australasian Leukaemia and Lymphoma Group (ALLG)
R. Bell, The Geelong Hospital, Geelong; R. Blum, Bendigo Hospital, Bendigo; W. I. Burns, St Vincent's Hospital, Fitzroy Melbourne; S. Deveridge, Newcastle Mater Hospital, Newcastle; S. Durrant, Royal Brisbane Hospital, Herston Brisbana; V. Ganju, Frankston Hospital, Frankston; R. Herrmann, Royal Perth Hospital, Perth; N. Horvath, Royal Adelaide Hospital, Adelaide; I. Irving, Townsville General Hospital, Townsville; R. Lowenthal, Royal Hobart Hospital, Hobart; P. Marlton, Princess Alexandra Hospital–University of Queensland, Woolloongabba; J. Norman, Queen Elizabeth Hospital, Woodville; A. Schwarer, Alfred Hospital, Prahran; M. Wolf, Peter Maccallum, East Melbourne.
EORTC Lymphoma Group (LYG)
P. Carde, Institut Gustave Roussy, Villejuif, France; H. Eghbali, Institut Bergoni, Bordeaux, France; H. M. Khaled, National Cancer Institute, Cairo, Egypt; O. C. Leeksma, Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands; J. Lemmens, Algemeen Ziekenhuis Sint-Augustinus, Wilrijk, The Netherlands; A. Rosta, National Institute of Oncology, Budapest, Hungary; J. Thomas, U. Z. Gasthuisberg, Leuven, Belgium; U. Tirelli, Centro Di Riferimento Oncologico, Aviano, Italy; R. Tomsic, The Institute of Oncology, Ljubljana, Slovenia; B. de Valk, Onze Lieve Vrouwe Gasthuis, Amsterdam, The Netherlands; J. Walewski, Maria Sklodowska-Curie Memorial Cancer Centre, Warsaw, Poland; P. W. Wijermans, Leyenburg Ziekenhuis, The Hague, The Netherlands; R. Willemze, Leiden University Medical Centre, Leiden, The Netherlands.
Nordic Lymphoma Group (NLG)
R. Ekanger, Haukeland Hospital–University of Bergen, Bergen; M. Eriksson, Lund University Hospital, Lund, Sweden; M. Erlanson, Umea Universitet, Umea, Sweden; H. Hagberg, Akademiska Sjukhusut, Uppsala, Sweden; M. Hansen, Rigshospitalet, Copenhagen, Denmark; R. Heikkila, Central Hospital of Rogaland, Stavanger, Norway; H. Holte, Norwegian Radium Hospital, Oslo, Norway; M. Maissenholder, Regionsykehuset I Tromso, Tromso, Sweden; B. Ostenstad, Ullevaal Hospital, Oslo, Norway; M. Sender Baum, Sahlgrenska Sjukhuset, Goteborg, Sweden.
SA NH
P. Jacobs, Constantiaberg Medi-Clinic, Cape Town, South Africa; J. I. Raats, Delmar Medical Centre, Panorama; P. Ruff, Johannesburg General Hospital, Parktown, Johannesburg; D. Vorobiof, Sandton Oncology Centre, Sandton, Parklands.
SAKK
F. Cavalli, Ospedale San Giovanni, Bellinzona; C. Taverna, Universitaetsspital, Zurich.
Prepublished online as Blood First Edition Paper, July 27, 2006; DOI 10.1182/blood-2006-05-021113.
A complete list of the members of the Intergroup Collaborative Study (EORTC 20981) appears in “Appendix.”
Supported by grant 2U10 CA11488-28 (through 5U10 CA11488-35) from the National Cancer Institute (Bethesda, MD). The research project was supported by the Queen Wilhelmina Fund (The Netherlands).
The authors declare no competing financial interests.
M.H.J.v.O., R.K., R.E.M., M.W., E.K., and A.H. formed the writing committee designing the study and were principal investigators of the participating lymphoma groups; R.D.G. and A.J. performed the major part of the pathology review; M.v.t'V., A.V., and H.H. included the largest numbers of patients calculated per center; M.v.G. performed all statistical analyses; I.T. and C.R. performed all data analysis; and M.H.J.v.O. wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
The study drug was provided by F. Hoffmann-La Roche Pharmaceuticals Division. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.