Abstract
Intravenous infusion of autologous chronic lymphocytic leukemia (CLL) cells transduced with an adenovirus encoding CD40-ligand (CD154) caused rapid reductions in leukemia-cell counts and lymphnode size. We hypothesized that CD40-ligation via CD154 sensitized CLL cells to death-receptor-mediated apoptosis. We found that CD154-expressing cells induced expression of CD95 and the BH3-interacting-domain death agonist (Bid) in CLL, regardless of whether the leukemia cells had functional p53. Such treatment also induced p73, a p53-related transcription factor regulated by c-Abl kinase, and enhanced the sensitivity to fludarabine (F-ara-A) of CLL cells lacking functional p53. Transduction of CLL cells with an adenovirus encoding p73 also induced Bid and CD95 and enhanced the sensitivity to F-ara-A of p53-deficient CLL cells. However, inhibition of c-Abl with imatinib suppressed CD154-induced expression of p73, p73-induced expression of Bid and CD95, and blocked the sensitization of p53-deficient CLL cells to CD95-mediated or F-ara-A-induced apoptosis. Conversely, CLL cells transduced with an imatinib-resistant c-Abl mutant could be induced by CD154 to express p73 and Bid even when treated with imatinib. These results indicate that CD154 can sensitize leukemia cells to apoptosis via the c-Abl-dependent activation of p73 and mitigate the resistance of p53-deficient CLL cells to anticancer drug therapy.
Introduction
Transduction of chronic lymphocytic leukemia (CLL) B cells with replication-defective adenovirus encoding recombinant CD40-ligand (CD154) enhanced their antigen-presenting cell (APC) activity, allowing these leukemia cells to function as effective stimulator cells in autologous mixed lymphocyte reactions.1 Moreover, such cells could induce generation of cytotoxic T lymphocytes against both transfected and nontransfected autologous leukemia cells. This formed the rationale for clinical trials testing whether such transduced leukemia cells could induce therapeutic antileukemia immune responses in vivo.
Although intended to induce an adaptive immune response against leukemia-associated antigens, infusion of CD154-expressing leukemia cells caused rapid reductions in leukemia-cell counts and lymph node size of the treated patients.2 The kinetics of leukemia-cell clearance suggested that innate immune effector mechanisms contributed to the initial reduction in leukemia-cell counts following such treatment. Consistent with this notion, we found that both transduced and bystander CLL cells expressed extrinsic death receptors, Fas (CD95) and DR5, and acquired latent sensitivity to Fas/TRAIL-mediated apoptosis in vitro.2,3 The expression of ligands for these death receptors could be found on natural killer (NK) cells and activated CD4 T cells that were present in the blood of patients following infusion of autologous, CD154-transduced leukemia cells.2-4
Leukemia-cell expression of these death receptors could be mediated by p53. p53 can activate transcription and expression of a number of genes encoding proteins responsible for clearance of cells that experience nonrepairable genotoxic stress. Specifically, genes encoding CD95 and DR5 both have p53 binding elements in the promoter and/or first intron, allowing for their induced expression upon activation of p53.5,6 Moreover, both receptors are induced by γ-irradiation in a p53-dependent fashion (reviewed by Sheard7 ). Also, p53 has been implicated in the induction of Bid, a molecule that also is expressed in CLL cells stimulated by CD1544,8 and that can sensitize tumor cells to many anticancer drugs.9 Because CD40-ligation on CLL cells can induce p53,10 we hypothesized that p53 might play a role in the latent sensitization of CD154-treated CLL cells to Fas-mediated apoptosis. As such, the leukemia-cell expression of functional p53 may be critical for the expression of death receptors and/or latent sensitization to Fas/TRAIL-mediated apoptosis following CD154 gene therapy.
However, CLL cells can incur somatic deletions in the short arm of chromosome 17 and/or inactivating mutations in p53.11 This is associated with resistance to standard anticancer drugs and poor prognosis.12,13 Conceivably, loss of p53 function also could make leukemia cells incapable of expressing death receptors or in becoming sensitive to Fas/TRAIL-mediated apoptosis following treatment with CD154. As such, patients with leukemia cells harboring such defects may be resistant to CD154 gene therapy.
However, some of the patients treated in early clinical trials had leukemia cells that apparently lacked p53 function yet still experienced acute reductions in leukemia-cell counts following infusion of autologous, CD154-transduced leukemia cells. This implies that the acute clearance of leukemia cells following CD154 gene therapy is not dependent on functional p53. To examine the p53-dependency of the acquired latent sensitivity to Fas-mediated apoptosis following activation by CD154, we examined the in vitro responses to CD154 of CLL cells that did or did not have functional p53.
Materials and methods
Cells
We obtained blood from patients with CLL following written informed consent. The mononuclear cells were isolated by density centrifugation over Ficoll-Paque (Amersham Biosciences, Piscataway, NJ) and viably frozen in fetal bovine serum (FBS; Omega Scientific, Tarzana, CA) containing 10% DMSO. Chinese hamster ovary (CHO) cells and HeLa cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA). CHO cells that express human Fas-ligand (CHO-CD178) and HeLa cells that express human CD154 (HeLa-CD154) were as described.3,4
CD40 activation and treatment with fludarabine monophosphate (F-ara-A)
CLL cells were cocultured for 24 hours with HeLa or HeLa-CD154 cells in RPMI culture medium (Irvine Scientific, Santa Anna, CA) supplemented with 10% FBS (Gibco/Invitrogen, Carlsbad, CA), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco, Grand Island, NY) in 5% CO2 at 37°C. After 24 hours, CLL cells were separated from the adherent HeLa cells, placed in culture for 1 hour in 12-well plates to allow contaminating HeLa cells to readhere to the plastic surface. The nonadherent CLL cells were harvested, washed, and then cultured alone at 5 × 106 cells/mL for the indicated time(s). In some experiments, imatinib mesylate (imatinib) was added to the CLL cells 30 minutes prior to their initial exposure to HeLa or Hela-CD154 cells. Imatinib did not reduce the viability of CLL relative to that of control cultures. (For example, CLL cells at day 5 after CD40-activation had a mean viability of 70% ± 9% [SD, n = 7], whereas CLL cells cultured without imatinib had a mean viability of 70% ± 18% at day 5 after CD40 activation.) In some experiments, mouse L-cells engineered to express human CD32 were used as feeder cells. This allowed us to add a mouse IgG1 isotype-control monoclonal antibody (mAb) or an agonist anti-CD40 antibody (G28-514 ) to effect CD40-ligation of the CLL cells, as described.15 All time points of CD154 activation refer to days after completion of the 24-hour coculture of the CLL cells with HeLa-CD154 cells. For treatment with fludarabine monophosphate (F-ara-A), the CLL cells were cocultured for only 12 hours with HeLa or HeLa-CD154. After separation from the adherent HeLa cells, the CLL cells were treated for 48 hours in culture medium with or without 3 μM F-ara-A and then assessed for apoptosis by flow cytometry.
Ionizing radiation (IR)
For studies using ionizing radiation (IR), CLL cells were cultured at a density of 5 × 106 cells/mL and irradiated at a dose of 5 Gray (Gy) using a calibrated Cesium137 source. We examined for the induction of death receptors following CD154 treatment or IR on CD19+ CLL cells by flow cytometry using fluorochrome-conjugated mAb (CD95; BD-PharMingen, San Diego, CA) or unconjugated mAb (DR5; Alexis, Carlsbad, CA) developed with second-step fluorochrome-conjugated anti-mouse-IgG antibody (BD-PharMingen). In each case, an isotype control mAb of irrelevant specificity was used to monitor for nonspecific cytophillic binding. To compare expression levels of surface antigens, we calculated the mean fluorescence intensity ratio (MFIR) for each antigen. This is the ratio of the mean fluorescence of cells stained with the fluorochrome-conjugated, antigen-specific mAb, divided by the mean fluorescence intensity of cells stained with a fluorochrome-conjugated, isotype-control mAb of irrelevant specificity.
Immunoblot analyses
For preparation of whole-cell lysates, the cells were collected by centrifugation at 250g for 10 minutes at 4°C, washed once in ice-cold phosphate buffered saline (PBS), pH 7.4, and lysed via sonification in radioimmunoprecipitation (RIPA) buffer (50 mM Tris/HCl, pH 7.4, 1% Nonidet P-40, 0.25% Na-Deoxycholate, 0.1% SDS, 150 mM NaCl, 5 mM EDTA, 1 mM PMSF, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 10 μg/mL pepstatin) and incubated for 15 minutes on ice. The lysates were cleared from insoluble debris by centrifugation at 21 000g for 10 minutes at 4°C and the supernatants were stored at -80°C until examined. Fifty micrograms of protein lysate was loaded onto each lane of a 5% to 15% gradient SDS-polyacrylamide gel electrophoresis (PAGE) gel (BioRad, Hercules, CA) and transferred to a polyvinylidene fluoride microporous membrane (Millipore, Billerica, MA). Membranes were probed with anti-p73 antibody (clone 42916 ), anti-p53 (EMD Biosciences, San Diego, CA), anti-Flag M5 (Sigma, St Louis, MO), or anti-CD95 (BD-PharMingen), followed by goat anti-mouse IgG-horseradish peroxidase (HRP; Santa Cruz Biotechnology, Santa Cruz, CA), or by anti-Bid antibody (Cell Signaling Technology, Beverly, MA) followed by goat anti-rabbit IgG-HRP (Santa Cruz Biotechnology). Stripped membranes were probed with anti-β-actin (Sigma) followed by goat anti-mouse IgG-HRP to monitor for protein loading.
Apoptosis assays
Apoptosis of CLL cells was monitored by flow cytometry using 3,3′ dihexyloxacarbocyanine iodide (DiOC6) (Molecular Probes, Eugene, OR) and propidium iodide (PI; Molecular Probes), as described.17 CLL target cells were cocultured with CHO effector cells for 8 hours at a 1:10 ratio. To distinguish CHO cells from CLL cells, the CHO cells were labeled with PKH26 (Sigma) immediately prior to coculture. After 8 hours, the cells were stained with 40 nM DiOC6 for 15 minutes at 37°C and analyzed with a FACS-Calibur (Becton Dickinson, San Jose, CA). The percent specific killing was determined by subtracting the level of apoptosis observed in the target cell population cultured with CHO cells from the level of apoptosis observed in the target cell population cultured with CHO cells that expressed CD178.
Adenovirus vectors
To generate the adenovirus vector encoding the c-Abl mutant resistant to imatinib, we used the cDNA encoding a Flag-tagged mouse c-Abl carrying the T315I point mutation.18 The cDNA was cloned into the polylinker site of the pcDNA3 expression vector (Invitrogen, San Diego, CA). The EcoRI/DraI fragment, including the CMV promoter and the polyadenylation signal from pcDNA3, was cloned into the shuttle vector MCS(SK)pXCX219 and designated c-Abl (T315I) pXCX2. The cDNA for human TAp73α was placed into the shuttle vector MCS(SK)pXCX2 and designated TAp73α pXCX2. The c-Abl(T315I) pXCX2 and TAp73α pXCX2 were each cotransfected with pJM17 into 293 cells using the calcium phosphate method. Isolated adenovirus plaques were harvested and expanded by again infecting 293 cells. High titer adenovirus was obtained by anion exchange chromatography, as described.20,21 The virus titer typically was in the range of 1 × 1010 to 3 × 1010 plaque forming units (pfu) per milliliter.
Results
CD154 induces p53 and p53-target genes in CLL B cells
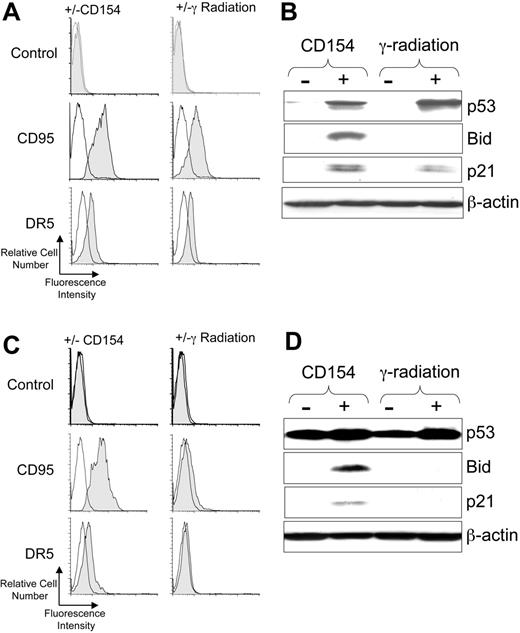
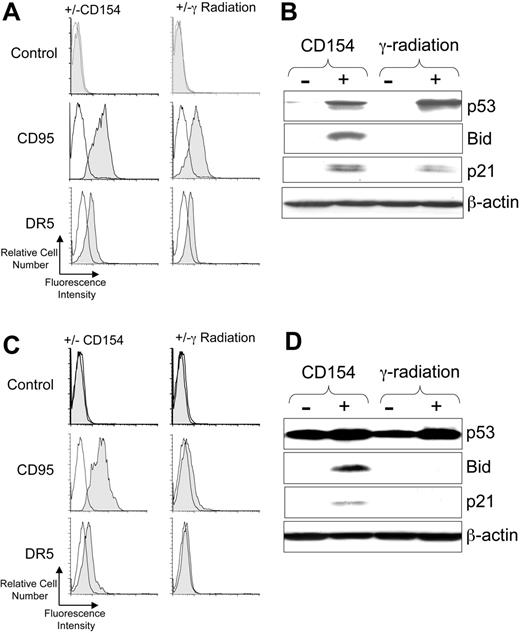
Ionizing radiation can induce activation of p53, which in turn can induce expression of CD95 and DR5.22,23 Consistent with this, we found that 5 Gy γ-irradiation induced expression of both CD95 and DR5 on CLL cells with functional p53 (Figure 1A). Similarly, coculture of CLL cells with HeLa-CD154, but not with HeLa lacking expression of CD154, induced expression of these death receptors within 24 hours of treatment (Figure 1A).
CD40-ligation of CLL cells induces p53 and p53-regulated genes. CLL cells were cocultured with HeLa cells (-) or HeLa cells expressing CD154 (+) or cultured alone without treatment (-) or after treatment with 5 Gy (γ-radiation). After 24 hours the CLL cells were harvested and examined via flow cytometry or immunoblot analyses. For panels A and C, cell-surface expression of death receptors on CD19+ CLL cells was monitored by flow cytometry. Representative histograms of CLL samples that were stained with fluorochrome-conjugated mAbs specific for an irrelevant specificity (control; top row), CD95 (middle row), or DR5 (bottom row), as indicated on the left margin, are shown. The shaded histogram depicts the fluorescence of CLL cells cocultured with HeLa-CD154 (left column) or following γ-radiation (right column), as indicated at the top of each column. The open histograms depict the fluorescence of stained control-treated CLL cells following culture with (left column) or without (right column) HeLa cells. For panels B and D, cell lysates were prepared from CLL cells that had been cocultured with HeLa cells lacking CD154 (-) or HeLa-CD154 (+), or cultured alone after no treatment (-) or after 5 Gy ionizing radiation (+), as indicated at the top of each panel. Fifty μg of each lysate were resolved via PAGE for immunoblot analyses using antibodies specific for p53, Bid, p21, or β-actin, as indicated on the right-hand margin of each immunoblot. In panels A and B are the results from analyzing CLL cells with functional p53, as indicated at the top of the figure. In panels C and D are the results obtained using CLL cells that had a monoallelic, nonfunctional p53 gene, confirmed by DNA sequencing to harbor an inactivating mutation (eg, Met237Ile), as indicated at the top of the figure.
CD40-ligation of CLL cells induces p53 and p53-regulated genes. CLL cells were cocultured with HeLa cells (-) or HeLa cells expressing CD154 (+) or cultured alone without treatment (-) or after treatment with 5 Gy (γ-radiation). After 24 hours the CLL cells were harvested and examined via flow cytometry or immunoblot analyses. For panels A and C, cell-surface expression of death receptors on CD19+ CLL cells was monitored by flow cytometry. Representative histograms of CLL samples that were stained with fluorochrome-conjugated mAbs specific for an irrelevant specificity (control; top row), CD95 (middle row), or DR5 (bottom row), as indicated on the left margin, are shown. The shaded histogram depicts the fluorescence of CLL cells cocultured with HeLa-CD154 (left column) or following γ-radiation (right column), as indicated at the top of each column. The open histograms depict the fluorescence of stained control-treated CLL cells following culture with (left column) or without (right column) HeLa cells. For panels B and D, cell lysates were prepared from CLL cells that had been cocultured with HeLa cells lacking CD154 (-) or HeLa-CD154 (+), or cultured alone after no treatment (-) or after 5 Gy ionizing radiation (+), as indicated at the top of each panel. Fifty μg of each lysate were resolved via PAGE for immunoblot analyses using antibodies specific for p53, Bid, p21, or β-actin, as indicated on the right-hand margin of each immunoblot. In panels A and B are the results from analyzing CLL cells with functional p53, as indicated at the top of the figure. In panels C and D are the results obtained using CLL cells that had a monoallelic, nonfunctional p53 gene, confirmed by DNA sequencing to harbor an inactivating mutation (eg, Met237Ile), as indicated at the top of the figure.
CD154 induces p73 in CLL cells with or without functional p53. CLL cells were CD40-activated as described in “Materials and methods” and protein lysates were prepared at the indicated time points. Immunoblot membranes prepared from such samples were probed for p73, β-tubulin, or β-actin to monitor for protein loading, as indicated at the right of each immunoblot. (A) The immunoblot of a representative time course of p73 expression is shown. (B) Two independent immunoblots from 2 different CLL samples were examined by densitometry using NIH Image software. The value before CD40-ligation (before) was subtracted from all time points as background value and set to 0%. The maximum value on day 3 after CD40-ligation was set to 100%. All other values were calculated relative to the maximum value [(intensity on day x / maximum intensity) × 100]. The graph shows mean values plus or minus the standard deviation of signal intensity on the y-axis relative to the time after CD154 treatment (eg, day 0 [D0], day 1 [D1], or day 3 [D3]), as indicated on the x-axis. (C) CLL cells were classified as having functional (p53 +) or nonfunctional p53 (p53-) by DNA sequencing of the p53 gene and/or by the functional assay of their response to γ-irradiation. Two patient samples each of CLL cells that were p53+ (samples from patients 1 and 2) or p53-(samples from patients 3 and 4) were analyzed 24 hours after treatment with HeLa cells (-) or HeLa-CD154 cells (+), as indicated at the top of each lane of the immunoblots.
CD154 induces p73 in CLL cells with or without functional p53. CLL cells were CD40-activated as described in “Materials and methods” and protein lysates were prepared at the indicated time points. Immunoblot membranes prepared from such samples were probed for p73, β-tubulin, or β-actin to monitor for protein loading, as indicated at the right of each immunoblot. (A) The immunoblot of a representative time course of p73 expression is shown. (B) Two independent immunoblots from 2 different CLL samples were examined by densitometry using NIH Image software. The value before CD40-ligation (before) was subtracted from all time points as background value and set to 0%. The maximum value on day 3 after CD40-ligation was set to 100%. All other values were calculated relative to the maximum value [(intensity on day x / maximum intensity) × 100]. The graph shows mean values plus or minus the standard deviation of signal intensity on the y-axis relative to the time after CD154 treatment (eg, day 0 [D0], day 1 [D1], or day 3 [D3]), as indicated on the x-axis. (C) CLL cells were classified as having functional (p53 +) or nonfunctional p53 (p53-) by DNA sequencing of the p53 gene and/or by the functional assay of their response to γ-irradiation. Two patient samples each of CLL cells that were p53+ (samples from patients 1 and 2) or p53-(samples from patients 3 and 4) were analyzed 24 hours after treatment with HeLa cells (-) or HeLa-CD154 cells (+), as indicated at the top of each lane of the immunoblots.
Because CD40-ligation can induce p53,10 we reasoned that coculture with CD154-bearing cells also could induce CLL cells to activate p53. We monitored for activation of p53 and p53-target genes (eg, p2122 and Bid9 ) following γ-irradiation or treatment with CD154-bearing cells. We found either γ-radiation or CD154 induced activation of p53, which was not apparent in control-treated CLL cells (Figure 1B). Consistent with the induced activation of p53, we noted that either treatment also induced expression of p21. However, in contrast to CD154-treated CLL cells, the CLL cells treated with γ-radiation failed to express Bid (Figure 1B).
We examined the CLL-cell samples that lacked functional p53 (n = 13), many of which harbored deletions at 17p13.1. Consistent with these cells lacking functional p53,24 we found that radiation of such cells failed to induce CD95, DR5, or p21 (Figure 1C-D, and data not shown). One of the samples that failed to respond to γ-radiation had a monoallelic deletion at 17p13.1 and an inactivating Met237Ile mutation in the retained p53 allele (Figure 1C-D). This sample constitutively expressed high levels of dysfunctional p53 by immunoblot analysis (Figure 1D). CD154 treatment still could induce such p53-defective CLL cells to express Bid, p21, CD95, and DR5.
CD154 induces expression of the trans-activating alpha isoform of p73 in CLL
Another member of the p53 family, p73, also can induce expression of p53 target genes such as p21.25-27 In addition, p73 apparently plays a role in activation-induced T-cell death.28,29 As activation-induced cell death shares some features with latent sensitization to CD95-mediated apoptosis of CD154-treated CLL cells,3,30 we examined CLL cells for expression of p73 following exposure to CD154-bearing cells. CD154 specifically induced CLL cells to express a protein of 73 kDa that reacted with the anti-p73 antibody (Figure 2), consistent with the induced expression of the alpha isoform of p73 (TAp73α).18 This isoform of p73 contains a transactivation-domain (TA), reviewed by Moll and Slade.31 Activation of CLL cells via coculture with CD154-bearing cells induced expression of TAp73α in nearly all cases examined (n = 23), including most cases that lacked functional p53 (Figure 2C). Moreover, exposure to CD154-bearing cells induced all samples to express CD95, DR5, and Bid, including the samples that lacked functional p53 (Figure 1, and data not shown).
Imatinib inhibits the capacity of CD154 to induce expression of TAp73α, Bid, and CD95
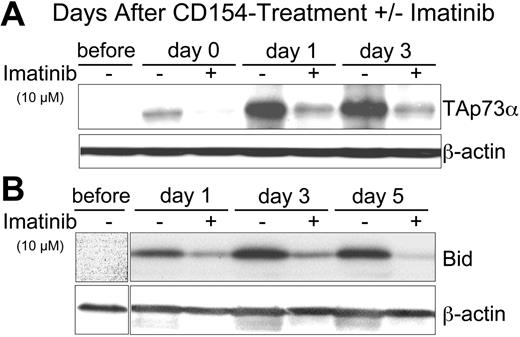
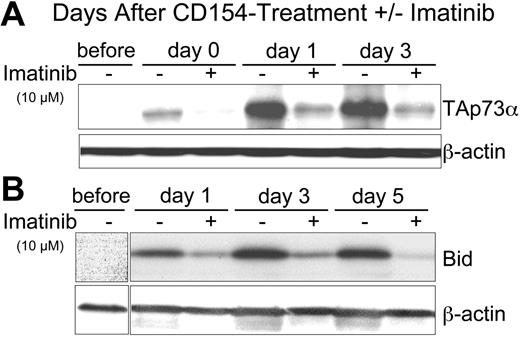
Expression of p73 is regulated at the transcriptional level by the transcription factor E2F-128 and at the posttranslational level by c-Abl kinase.16,32-34 Phosphorylation of p73 by c-Abl increases the half-life and activity of p73. Because the kinase activity of c-Abl can be inhibited by imatinib,35,36 we examined the effect of CD154 on CLL cells treated with this kinase inhibitor. We found that addition of imatinib to the CLL cells at the time of exposure to CD154-bearing cells could inhibit the induced expression of TAp73α in a dose-dependent fashion that was apparent even at day 3 following exposure to CD154-bearing cells (Figure 3A and data not shown). Similarly, we found that 10 μM imatinib could inhibit the CD154-induced expression of Bid (Figure 3B) and CD95 (data not shown). However, imatinib failed to inhibit the CD154-induced expression of FADD, caspase 8, or Flip in any of the CLL samples tested (data not shown). These data suggest that c-Abl kinase plays a role in the CD154-induced expression and activation of TAp73α.
To examine whether expression of TAp73α was sufficient to induce expression of Bid and/or CD95, we transfected CLL cells with a replication-defective adenovirus vector encoding TAp73α (Ad-TAp73α) or a control adenovirus vector (Ad-blank). Transduction of CLL cells with Ad-TAp73α, but not with Ad-blank, resulted in high-level expression of TAp73α that did not require culture with CD154-bearing cells (and presumably also activation of c-Abl kinase) (Figure 4A). Expression of TAp73α was associated with induced expression of CD95 and Bid, even in CLL cells lacking functional p53 (eg, samples from patient no. 9; Figure 4 and data not shown). These findings suggest that TAp73α was sufficient to induce CLL cells to express CD95 and Bid and that this did not require functional p53.
Transduction of CLL cells with Ad-TAp73α, but not Ad-blank, also induced low-level surface expression of CD95 (Figure 4B, shaded histogram, right panel). Addition of imatinib at 10 μM to the CLL cells prior to transduction with Ad-TAp73α blocked the p73-induced expression of CD95 (Figure 4B, open dark-lined histogram, right panel).
Imatinib inhibits the capacity of CD154 to induce CLL-cell expression of TAp73 α and Bid. (A) Cell lysates were prepared from CLL cells prior to culture (before) or at 0 (day 0), 1 (day 1), 3 (day 3), or 5 days (day 5) after 24-hour culture with HeLa-CD154 in media without (-) or with (+) 10 μM imatinib. Fifty micrograms of protein from each lysate was loaded onto separate lanes for PAGE and immunoblot analyses, as indicated at the top of each panel. Immunoblots were stained with antibodies specific for p73 (TAp73α) or β-actin (to control for protein loading), as indicated at the right of each immunoblot. (B) Immunoblots as in panel A were stained with antibodies specific for Bid or β-actin, as indicated at the right of each immunoblot.
Imatinib inhibits the capacity of CD154 to induce CLL-cell expression of TAp73 α and Bid. (A) Cell lysates were prepared from CLL cells prior to culture (before) or at 0 (day 0), 1 (day 1), 3 (day 3), or 5 days (day 5) after 24-hour culture with HeLa-CD154 in media without (-) or with (+) 10 μM imatinib. Fifty micrograms of protein from each lysate was loaded onto separate lanes for PAGE and immunoblot analyses, as indicated at the top of each panel. Immunoblots were stained with antibodies specific for p73 (TAp73α) or β-actin (to control for protein loading), as indicated at the right of each immunoblot. (B) Immunoblots as in panel A were stained with antibodies specific for Bid or β-actin, as indicated at the right of each immunoblot.
Imatinib inhibition of C-Abl kinase accounts for its ability to inhibit expression of TAp73 α and Bid following CD40-ligation
The results from experiments represented in Figure 3 and Figure 4A-B suggest that c-Abl kinase is responsible for the induced expression of Bid and CD95. However, imatinib can inhibit the activity of kinases other than c-Abl, such as c-kit or platelet-derived growth factor receptor.36,37 To examine whether c-Abl indeed was required for CD154-induced activation and/or expression of TAp73α and/or Bid, we transduced CLL cells with an adenovirus encoding a mutant form of c-Abl, Ad-c-Abl (T315I), that was resistant to inhibition by imatinib.38 This construct had a Flag tag, allowing for its identification in transfected cells via immunoblot analyses using anti-Flag antibodies. CLL cells were transduced with a control adenovirus (Ad-blank) or with the mutant Ad-c-Abl (T315I) and then activated by coculture with agonist anti-CD40 mAb presented on CD32-bearing L cells, as described.15 In contrast to the CLL cells transduced with Ad-blank, the Ad-c-Abl (T315I)-transduced leukemia cells expressed protein reactive with the anti-Flag antibody (Figure 4C, third row). CLL cells transduced with either vector failed to express high-level TAp73 or Bid upon culture with CD32 L cells unless agonist anti-CD40 mAb was added to the culture media. While addition of imatinib at 10 μM to the cultures inhibited the anti-CD40 mAb-induced expression of TAp73 or Bid in CLL cells transduced with Ad-blank, imatinib failed to suppress the induced-expression of TAp73 or Bid in CLL cells transduced with Ad-c-Abl (T315I) (Figure 4C, first and second rows).
Imatinib inhibits CD154-induced sensitization of CLL cells to apoptosis
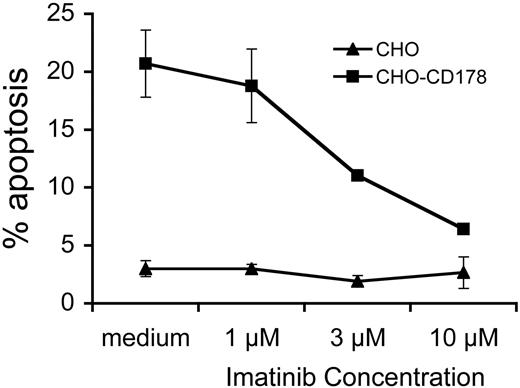
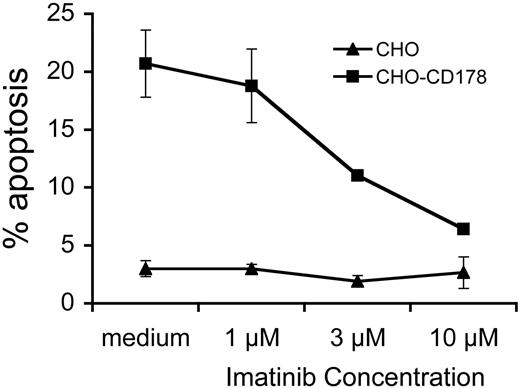
We examined whether imatinib could inhibit the sensitization to CD95-mediated apoptosis of CLL cells following CD40-ligation. After exposure to CD154, CLL cells acquire latent sensitivity to CD95-mediated apoptosis,3 regardless of whether the leukemia cells have functional p53 (data not shown). Addition of imatinib to CLL cells at the time of CD154-activation did not affect the viability of CLL cells over that of control cultures (data not shown). However, such treatment rendered CD154-activated CLL cells insensitive to apoptosis induced by cells bearing the ligand for CD95, namely CHO-CD178 cells, in a dose-dependent fashion (Figure 5). Maximum inhibition was achieved at 10 μM imatinib if present during the time when the CLL cells were exposed to the CD154-HeLa cells. Addition of imatinib at later time points, such as when the CLL cells were exposed to CHO-CD178 cells, did not reduce the levels of apoptosis observed in the CD154-treated CLL cells (data not shown). As such, imatinib could block the sensitization of CLL cells to CD95-mediated apoptosis, but not the actual killing of the CLL cells after they had been stimulated by CD154.
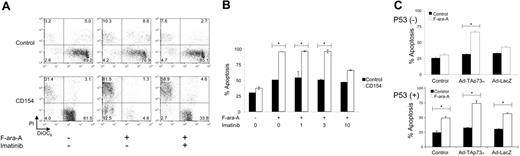
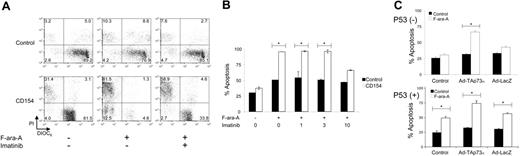
We examined whether CD154 might enhance the sensitivity of p53-deficient CLL cells to anticancer drugs. For this we examined the sensitivity of CLL cells to F-ara-A, a drug that commonly is used in the treatment of CLL and that apparently functions via a p53-dependent mechanism.39 p53-deficient CLL cells were exposed to HeLa (control) or CD154-HeLa cells (CD154) for 12 hours in cultured medium that did (+), or did not (-), contain 10 μM imatinib. The leukemia cells were removed from the stimulator cells and then tested for sensitivity to 3 μM F-ara-A. After culture in medium with or without F-ara-A, the CLL cells were stained with DiOC6 and PI and examined via flow cytometry. A representative experiment is presented in Figure 6A. Most of the CLL cells previously cultured on HeLa cells remained viable, staining brightly with DiOC6, but not with PI (lower right-hand quadrant of each panel) regardless of whether they were treated with medium alone (Figure 6A, top row, left panel) or medium containing 3 μM F-ara-A (Figure 6A, top row, middle panel) or 3 μM F-ara-A and 10 μM imatinib (Figure 6A, top row, right panel). However, F-ara-A treatment of CLL cells that had been cultured on HeLa-CD154 cells experienced a marked reduction in the relative proportions of viable cells that stained brightly with DiOC6 and excluded PI (Figure 6A, bottom row, middle panel) relative to that noted in HeLa-CD154-treated CLL cells cultured in medium alone (Figure 6A, bottom row, left panel). In contrast, CLL cells treated with 10 μM imatinib at the time of their coculture with HeLa-CD154 cells had an increased proportion of cells that stained brightly with DiOC6 and excluded PI following treatment with F-ara-A (Figure 6A, bottom row, right panel). In another experiment, CLL-cell samples lacking functional p53 (n = 2) were stimulated for 12 hours by coculture with HeLa or HeLa-CD154 (CD154) cells in the presence or absence of imatinib at various concentrations (Figure 6B). The cells were removed from the stimulator cells and then cultured alone in media with or without 3 μM F-ara-A. After 48 hours, the cells were examined for apoptosis by flow cytometry. CD154-activated CLL cells had significantly higher levels of apoptosis when treated with F-ara-A (Figure 6B, open bars) than did control HeLa-treated CLL cells (Figure 6B, black bars), which were insensitive to F-ara-A in vitro. However, CLL cells exposed to CD154-HeLa cells in medium containing 10 μM imatinib did not have significantly higher levels of F-ara-A-induced apoptosis than did control-treated CLL cells. Treatment with lower concentrations of imatinib did not have this effect (Figure 6B).
Imatinib inhibits the capacity to induce CLL-cell expression of Bid and CD95 except in CLL cells made to express imatinib-resistant mutant C-Ab. (A) CLL cells were transduced with an adenovirus encoding TAp73α (Ad-TAp73α) or lacking a transgene (Ad-blank), as indicated at the bottom of the panel. At 24 hours after the start of transduction, the CLL cells were isolated and cultured in fresh medium for another 48 hours. Cell lysates were prepared for immunoblot analyses using antibodies specific for p73 (TAp73α), CD95, Bid, or β-actin, as indicated on the right of each immunoblot. (B) CLL cells were transduced with an adenovirus expressing p73 (Ad-TAp73α) or with an adenovirus lacking transgene expression (Ad-blank) in the presence or absence of imatinib at 10 μM. After 48 hours the CLL cells were washed and cultured in fresh medium for another 24 hours and then examined for expression of surface CD95 by flow cytometry. Representative histograms show the expression of CD95 of Ad-blank- or Ad-TAp73α-transduced CLL cells cultured without (filled gray) or with (thick line) imatinib, as indicated at the top of each histogram. The thin lined open histogram corresponds to that of transduced CLL cells cultured without imatinib and stained with an isotype control mAb of irrelevant specificity. (C) CLL cells were transduced with an adenovirus encoding the Flag-tagged, imatinib-resistant c-Abl (Ad-c-Abl (T315I)) or Ad-blank, as indicated at the top of the immunoblot. Twenty-four hours later the CLL cells were washed extensively and then cocultured with CD32-expressing mouse L-cells and an isotype control mAb (control) or the agonist anti-CD40 mAb (α-CD40), in media containing (+)or lacking (-) imatinib at 10 μM, as indicated at the bottom of the panel. Twenty-four hours later, the CLL cells were isolated and used to prepare cell lysates. Fifty μg of each lysate was loaded onto separate lanes of a PAGE gel for immunoblot analyses. Membranes were probed with antibodies specific for p73, Bid, the Flag tag (c-Abl (α-Flag)), or β-actin, as indicated to the right of each blot.
Imatinib inhibits the capacity to induce CLL-cell expression of Bid and CD95 except in CLL cells made to express imatinib-resistant mutant C-Ab. (A) CLL cells were transduced with an adenovirus encoding TAp73α (Ad-TAp73α) or lacking a transgene (Ad-blank), as indicated at the bottom of the panel. At 24 hours after the start of transduction, the CLL cells were isolated and cultured in fresh medium for another 48 hours. Cell lysates were prepared for immunoblot analyses using antibodies specific for p73 (TAp73α), CD95, Bid, or β-actin, as indicated on the right of each immunoblot. (B) CLL cells were transduced with an adenovirus expressing p73 (Ad-TAp73α) or with an adenovirus lacking transgene expression (Ad-blank) in the presence or absence of imatinib at 10 μM. After 48 hours the CLL cells were washed and cultured in fresh medium for another 24 hours and then examined for expression of surface CD95 by flow cytometry. Representative histograms show the expression of CD95 of Ad-blank- or Ad-TAp73α-transduced CLL cells cultured without (filled gray) or with (thick line) imatinib, as indicated at the top of each histogram. The thin lined open histogram corresponds to that of transduced CLL cells cultured without imatinib and stained with an isotype control mAb of irrelevant specificity. (C) CLL cells were transduced with an adenovirus encoding the Flag-tagged, imatinib-resistant c-Abl (Ad-c-Abl (T315I)) or Ad-blank, as indicated at the top of the immunoblot. Twenty-four hours later the CLL cells were washed extensively and then cocultured with CD32-expressing mouse L-cells and an isotype control mAb (control) or the agonist anti-CD40 mAb (α-CD40), in media containing (+)or lacking (-) imatinib at 10 μM, as indicated at the bottom of the panel. Twenty-four hours later, the CLL cells were isolated and used to prepare cell lysates. Fifty μg of each lysate was loaded onto separate lanes of a PAGE gel for immunoblot analyses. Membranes were probed with antibodies specific for p73, Bid, the Flag tag (c-Abl (α-Flag)), or β-actin, as indicated to the right of each blot.
Imatinib inhibits CD154-induced sensitization of CLL cells to CD95-mediated apoptosis. CLL cells were cultured for 24 hours on CD154-bearing adherent cells in media containing 0 μM (medium), 1 μM, 3 μM, or 10 μM imatinib, as indicated at the bottom of the graph. On day 5, the CLL cells were isolated and then cultured with CHO (▴) or CHO-CD178 (▪) effector cells. The percentage of CLL cells undergoing apoptosis after 24 hours is indicated on the y axis (% apoptosis). This graph provides a representative assay performed twice on the CLL cells of one patient. Each data point is the mean plus or minus the standard error of the 2 assays.
Imatinib inhibits CD154-induced sensitization of CLL cells to CD95-mediated apoptosis. CLL cells were cultured for 24 hours on CD154-bearing adherent cells in media containing 0 μM (medium), 1 μM, 3 μM, or 10 μM imatinib, as indicated at the bottom of the graph. On day 5, the CLL cells were isolated and then cultured with CHO (▴) or CHO-CD178 (▪) effector cells. The percentage of CLL cells undergoing apoptosis after 24 hours is indicated on the y axis (% apoptosis). This graph provides a representative assay performed twice on the CLL cells of one patient. Each data point is the mean plus or minus the standard error of the 2 assays.
To examine whether this effect is due to p73, CLL cells samples lacking p53 (p53 (-)) or having functional p53 (p53 (+)) were each transduced with Ad-TAp73α or Ad-lacZ, a control adenovirus vector. Following transduction, the cells were cultured in medium with or without 3 μM F-ara-A. CLL cells with functional p53 had significantly higher proportions of apoptotic cells following treatment with F-ara-A (Figure 6C, lower panel open bars) than such CLL cells cultured in medium alone (Figure 6C, lower panel, black bars), regardless of whether or not the cells were transfected with either of the adenovirus vectors (Figure 6C). In contrast, CLL cells lacking functional p53 did not have significantly higher proportions of apoptotic cells when cultured with F-ara-A rather than in medium alone (Figure 6C, top panel, black columns marked “Control”). Infection of these CLL cells with Ad-lacZ did not increase their susceptibility to apoptosis induced by F-ara-A. However, transfection of such CLL cells with Ad-TAp73α enhanced their sensitivity to the F-ara-A such that a significantly higher proportion of cells underwent apoptosis following treatment with F-ara-A than following culture in medium alone (Figure 6C, top panel, middle open bar).
Discussion
While expression of CD95 obviously is essential for CD95-mediated apoptosis, it was not sufficient to sensitize leukemia cells to CD95-mediated apoptosis immediately following CD154 stimulation. However, over time such CLL cells become increasingly sensitive to CD95-mediated apoptosis through a process that apparently depended on the induced expression of Bid.4,40 In prior studies we found that Bid, which is newly expressed in CD154-stimulated CLL cells, was cleaved following CD95-ligation, indicating that it might contribute to the mechanism(s) responsible for apoptosis.4,8 Because p53 can induce expression of Bid,9 at least in some cell types,41 we hypothesized that the induced-expression of this proapoptotic member of the bcl-2 family in CLL was dependent on functional p53. Consistent with this, we found that CD154 induces CLL cells to express p53 and the p53-target genes CD95, DR5, p21, and Bid.
However, we found that levels of ionizing radiation capable of inducing p53 and p53-dependent target genes failed to induce expression of Bid in CLL cells (Figure 1). Also, CLL cells lacking functional p53 still were induced to express Bid and to acquire sensitivity to CD95-mediated apoptosis following coculture with CD154-bearing cells (Figures 1 and 5, and data not shown). As such, functional p53 appears to be neither necessary nor sufficient to induce Bid or to prime leukemia cells for CD95-mediated apoptosis.
Activation by CD154 and induction of p73 enhances the sensitivity of p53-deficient CLL cells to F-Ara-A. (A) CLL cells with nonfunctional p53 were exposed to HeLa cells (control) or CD154-HeLa cells (CD154) with (+) or without (-) 10 μM imatinib and then cultured alone with (+) or without (-) 3 μM F-ara-A, as indicated at the bottom of the panel. The cells were stained with DiOC6 (x-axis) and PI (y-axis) and then examined via flow cytometry. The numbers in each quadrant represent the relative proportion of cells found within each gated compartment. (B) CLL samples (n = 2) with nonfunctional p53 were exposed to HeLa (control, ▪) or CD154-HeLa (CD154, □) with various concentrations of imatinib, as indicated at the bottom of the panel in μM. The cells were then isolated and cultured without (-) or with (+)3 μM F-ara-A, as indicated at the bottom of the panel. Each bar represents the mean proportion of apoptotic CLL cells, as indicated on the y-axis, plus or minus standard deviation. Bars with asterisks indicate that the difference in the relative levels of apoptosis observed for control-treated cells (▪) or CD154-treated cells (□) is statistically significant (P < .05). (C) CLL cells with nonfunctional (p53-, top panel; n = 2) or functional p53 (p53+, bottom panel; n = 2) were transduced with Ad-TAp73α or Ad-LacZ or remained untransduced (control), as indicated at the bottom of panel. Twenty-four hours later the CLL cells were washed extensively and cultured for another 48 hours in media (▪) or in the presence of 3 μM F-ara-A (□). At the end of this incubation period the proportion of apoptotic cells was assessed via flow cytometry after staining the cells with DiOC6 and PI. Each bar represents the mean relative proportion of apoptotic CLL cells, as indicated on the y-axis, plus or minus the standard deviation. Bars with asterisks indicate that the difference in the relative levels of apoptosis observed for control-treated CLL cells (▪) versus F-ara-A-treated CLL cells (□) is statistically significant (P < .05).
Activation by CD154 and induction of p73 enhances the sensitivity of p53-deficient CLL cells to F-Ara-A. (A) CLL cells with nonfunctional p53 were exposed to HeLa cells (control) or CD154-HeLa cells (CD154) with (+) or without (-) 10 μM imatinib and then cultured alone with (+) or without (-) 3 μM F-ara-A, as indicated at the bottom of the panel. The cells were stained with DiOC6 (x-axis) and PI (y-axis) and then examined via flow cytometry. The numbers in each quadrant represent the relative proportion of cells found within each gated compartment. (B) CLL samples (n = 2) with nonfunctional p53 were exposed to HeLa (control, ▪) or CD154-HeLa (CD154, □) with various concentrations of imatinib, as indicated at the bottom of the panel in μM. The cells were then isolated and cultured without (-) or with (+)3 μM F-ara-A, as indicated at the bottom of the panel. Each bar represents the mean proportion of apoptotic CLL cells, as indicated on the y-axis, plus or minus standard deviation. Bars with asterisks indicate that the difference in the relative levels of apoptosis observed for control-treated cells (▪) or CD154-treated cells (□) is statistically significant (P < .05). (C) CLL cells with nonfunctional (p53-, top panel; n = 2) or functional p53 (p53+, bottom panel; n = 2) were transduced with Ad-TAp73α or Ad-LacZ or remained untransduced (control), as indicated at the bottom of panel. Twenty-four hours later the CLL cells were washed extensively and cultured for another 48 hours in media (▪) or in the presence of 3 μM F-ara-A (□). At the end of this incubation period the proportion of apoptotic cells was assessed via flow cytometry after staining the cells with DiOC6 and PI. Each bar represents the mean relative proportion of apoptotic CLL cells, as indicated on the y-axis, plus or minus the standard deviation. Bars with asterisks indicate that the difference in the relative levels of apoptosis observed for control-treated CLL cells (▪) versus F-ara-A-treated CLL cells (□) is statistically significant (P < .05).
Activation-induced cell death (AICD) of T lymphocytes also does not require functional p53, as shown in various knock-out studies.42 Instead, another member of the p53 family, namely p73, has been found to play a critical role in T-cell AICD.28,29 The gene structure of p73 is highly complex, encoding the transactivation (TA) competent isoforms, called TAp73, and isoforms that lack the N-terminal transactivation domain, collectively termed ΔNp73. Alternative splicing at the C-terminus gives rise to at least 9 different transcripts, with the α-transcript being full-length (reviewed by Moll and Slade31 ). While ectopic expression of TAp73 has similar effects in transactivation and apoptosis as does p53,25,31 ΔNp73 can inhibit the function of both p53 and TAp73.43,44 Expression of TAp73, as well as ΔNp73, has been reported in CLL cells.45,46 Because the process of latent sensitization to CD95-mediated apoptosis of CD40-activated CLL cells has some features in common with T-cell AICD, we hypothesized that TAp73 also may be induced by CD40 activation and that this factor could contribute to observed changes in CLL cells following exposure to CD154-bearing cells.
We found that CD40-activation induces high-level leukemia-cell expression of the α-isoform of TAp73 (TAp73α). Using an antibody specific for the N-terminus of p73 that is not present in ΔNp73, we found that CD154 induced CLL cells to express an isoform of p73 with the 73 kDa molecular size expected for that of TAp73α (Figure 2), but not other C-terminal isoforms of TAp73, which have lower molecular sizes. Although the induced expression of TAp73 is associated with apoptosis of some cell types,25 expression of TAp73 in CLL does not directly result in apoptosis without ligation of the extrinsic death receptors. Nonetheless, the induced expression of TAp73 following CD154 activation of CLL cells may contribute to cell-cycle arrest, as has been noted for other cell types.47 This most likely is due to the capacity of TAp73α to induce expression of the cell-cycle inhibitor p21.27 Consistent with this notion, we found that CD154 induces CLL-cell expression of p21, even in leukemia cells that lacked functional p53.
The expression and activity of TAp73 is regulated by the transcription factor E2F-1 and by the c-Abl protein kinase.31 The c-Abl kinase regulates TAp73 protein abundance by phosphorylating p73 at Tyr99 and by direct protein-protein interaction of the SH2-domain of c-Abl with phosphorylated p73.16,32-34 Two lines of evidence suggest that CD40 activation might induce TAp73α in CLL cells via a c-Abl kinase pathway or pathways: (1) inhibition of c-Abl kinase with imatinib during CD40-ligation also inhibited the induced expression of TAp73α (Figure 3A); and (2) expression of a point mutant of c-Abl, c-Abl (T315I), which is resistant to imatinib,38 enabled CLL cells to express activated TAp73α in response to CD40-ligation, even in the presence of imatinib (Figure 4C). The latter observation excludes the possibility that imatinib inhibits CD154-induced expression of TAp73 by inhibiting kinases other than c-Abl.
TAp73 shares a high degree of homology with p53, especially in the DNA-binding domain.26 Moreover, TAp73 can regulate many genes that are regulated by p53.25-27 Because CD154 stimulation could induce expression of p53-dependent genes, even in leukemia cells that lacked functional p53, we reasoned that the c-Abl-dependent expression of TAp73 following CD40-ligation might be responsible for the expression of the proapoptotic proteins encoded by such genes in CLL. We monitored the expression levels of proteins that are involved in CD95-mediated apoptosis (eg, CD95, FADD, caspase 8, Flip, Dap3, and Bid) in CLL cells stimulated by CD154-bearing cells in the presence of imatinib. Although we did not observe any changes in the induced expression levels of FADD, caspase 8, Flip, or DAP3 (data not shown), we found that imatinib could inhibit the induced expression of Bid and CD95 in CLL cells (Figure 4, and data not shown). The specificity of this inhibition was demonstrated in the studies using Ad-c-Abl (T315I), which encoded a mutant c-Abl resistant to imatinib. Transduction of CLL cells with this adenovirus, but not with a control adenovirus, enabled CLL cells to express Bid upon CD154 activation, even in the presence of imatinib (Figure 4C). This indicates that Bid is regulated in a c-Abl-dependent manner, presumably indirectly in response to the c-Abl-regulated expression of TAp73α. Consistent with this notion, CLL cells transduced with Ad-TAp73α that expressed high levels of TAp73α also expressed Bid and low levels of CD95 independent of CD40-ligation (Figure 4A-B). Nevertheless, expression of CD95 by CLL cells transduced with Ad-TAp73α still could be inhibited by imatinib (Figure 4B), implying that the forced expression of TAp73α still was dependent on c-Abl kinase for its activity. These studies suggest that TAp73α is at least partially involved in the induced expression of CD95 on CLL cells following CD154 stimulation. Consistent with this notion is the recent finding that TAp73 can regulate expression of CD95 in hepatocellular carcinoma cells via the intronic p53 enhancer.48 However, even though imatinib could completely block the induced expression of Bid, this drug could not entirely suppress the capacity of CD40-ligation to induce expression of CD95 on CLL cells, even on leukemia cells that lacked functional p53 (data not shown). As such, CD154 activation apparently also can induce expression of CD95 via a pathway, or pathways, that is independent of p53, TAp73, or c-Abl kinase.
A previous study in CLL cells found expression of the transactivation domain-deficient isoform of p73, namely ΔNp73.46 This isoform can inhibit the transactivation and apoptotic function of p53 and TAp73α.43,44 Although the anti-p73 antibody that was used in our study cannot detect these ΔNp73 isoforms, our data suggest that ΔNp73 does not play a functional role in induced expression of Bid following CD40 activation. TAp73α and Bid are induced in the initial 24 hours after CD40-ligation and increase over time (Figure 2A-B). Sustained expression of Bid in CLL cells also was observed in vivo in the bystander leukemia cells of patients treated with autologous, Ad-CD154-transduced CLL cells.4 Moreover, in contrast to the negligible expression of Bid in pretreatment CLL cells, CLL cells isolated from such patients maintained high-level expression of Bid for at least 2 weeks following a single bolus infusion of autologous, Ad-CD154-transduced CLL cells.4
Bid allows for crosstalk between the extrinsic death receptors and the so-called intrinsic mitochondrial apoptotic pathway.49,50 In some cell types Bid is necessary to induce effective CD95-mediated apoptosis.40 In 2 recent studies we hypothesized that this Bid-mediated crosstalk might be necessary for the process of latent sensitization to CD95-mediated apoptosis.4,51 In the present study we tested this hypothesis by ligating CD95 on CLL B cells that were inhibited from expressing Bid by treatment with imatinib. This correlated with a significant and dose-dependent inhibition of latent sensitization to CD95-mediated apoptosis of CLL samples even at day 5 after CD40-ligation (Figure 5), when high-level expression of CD95 was observed.
In contrast to p53, p73 has not been implicated in tumor suppression, but it has been implicated in apoptosis. TAp73α apparently functions to enhance the sensitivity of cancer cells to the cytotoxic effects of anticancer drugs.52,53 Because expression of Bid also appears to contribute to the sensitivity of cancer cells to drug-induced apoptosis,9 we hypothesized that the expression of TAp73α and/or Bid following CD154-activation also might sensitize CLL cells to the cytotoxic effects of anticancer drugs that ordinarily require functional p53. Two lines of evidence in our study support this hypothesis. First, CD154 activation of CLL cells lacking functional p53 significantly enhanced their sensitivity to the cytotoxic effects of subsequent treatment with F-ara-A. Second, treatment of CLL cells with imatinib during the time of CD154 activation abrogated this effect (Figure 6A-B).
In contrast to the results presented here, previous studies found that CD154 actually could decrease the sensitivity of CLL cells to apoptosis induced by F-ara-A.54,55 However, in these studies the CLL cells were treated with F-ara-A and CD154 simultaneously rather than in a series. Instead, we examined the sensitivity to drug-induced apoptosis of CLL cells after they had been stimulated by CD154, as was done in another prior study,56 which found that prior treatment with CD154 actually could enhance the sensitivity of CLL cells to the cytotoxic activity of F-ara-A. However, it was not known whether the CLL-cell samples examined in this earlier study lacked functional p53.
From the results of the present study, it appears that TAp73α and/or Bid can cooperate with F-ara-A in inducing apoptosis of CLL cells. Indeed, p53-deficient CLL cells transfected with Ad-TAp73α had significantly higher levels of apoptosis after treatment with F-ara-A than when they were transfected with a control adenovirus vector, Ad-lacZ (Figure 6C). Conceivably, strategies such as CD154 gene therapy, which can induce tumor-cell activation of TAp73, may restore the in vivo sensitivity to drug-induced apoptosis of CLL cells that have acquired inactivating mutations and/or deletions in the genes encoding proteins of the p53 pathway. If so, then CD154 gene therapy potentially might benefit patients with p53-deficient CLL not only due to its intended capacity for inducing antileukemia immunity, but also because of its potential to resensitize leukemia cells to the cytotoxic activity of anticancer drug therapy.
Prepublished online as Blood First Edition Paper, June 1, 2006; DOI 10.1182/blood-2006-04-017749.
Supported in part by PO1 CA881 534 and R37 CA49 870 from the National Institutes of Health and by Dutch Cancer Foundation and René Vogels Foundation grants (A.P.K.).
The authors declare no competing financial interests.
F.D. and A.P.K. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We are grateful to Dr Laura Rassenti, Esther Avery, Monica Cook, Lang Huynh, and Traci Toy for their technical assistance.

![Figure 2. CD154 induces p73 in CLL cells with or without functional p53. CLL cells were CD40-activated as described in “Materials and methods” and protein lysates were prepared at the indicated time points. Immunoblot membranes prepared from such samples were probed for p73, β-tubulin, or β-actin to monitor for protein loading, as indicated at the right of each immunoblot. (A) The immunoblot of a representative time course of p73 expression is shown. (B) Two independent immunoblots from 2 different CLL samples were examined by densitometry using NIH Image software. The value before CD40-ligation (before) was subtracted from all time points as background value and set to 0%. The maximum value on day 3 after CD40-ligation was set to 100%. All other values were calculated relative to the maximum value [(intensity on day x / maximum intensity) × 100]. The graph shows mean values plus or minus the standard deviation of signal intensity on the y-axis relative to the time after CD154 treatment (eg, day 0 [D0], day 1 [D1], or day 3 [D3]), as indicated on the x-axis. (C) CLL cells were classified as having functional (p53 +) or nonfunctional p53 (p53-) by DNA sequencing of the p53 gene and/or by the functional assay of their response to γ-irradiation. Two patient samples each of CLL cells that were p53+ (samples from patients 1 and 2) or p53-(samples from patients 3 and 4) were analyzed 24 hours after treatment with HeLa cells (-) or HeLa-CD154 cells (+), as indicated at the top of each lane of the immunoblots.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/10/10.1182_blood-2006-04-017749/4/m_zh80220603580002.jpeg?Expires=1767790787&Signature=G4RlT8RTA9nKC5OJ36y1YybOe7NV63NM9WsRkIvHutSchm3Xgz-d71V~AWw7dDoj7yRrtsN84wwMJVkbycApqy8dPiTylOhGWfSau0X4-J28Hq-0J9d~VsxYtohrDZhmAuak5zAuYJhfn6Z5KkuLnzX410UJJSammVRPgBzSNemQLXq1BAA4mVwhaO1fM2ewwgsnSVe3cQqELfZzRYpQ7rYzfbIaLORhjKziJlT~KrMmUBnVJK2hskrr1tUHBeeM0-OnwDl55u5wZo9dJvj1NPLqJV7BxMtuaB8danDe5nrKD1wWvXyUN21dNXrGVELo71eSd2S~Qb6RM~LvfYEOFA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 2. CD154 induces p73 in CLL cells with or without functional p53. CLL cells were CD40-activated as described in “Materials and methods” and protein lysates were prepared at the indicated time points. Immunoblot membranes prepared from such samples were probed for p73, β-tubulin, or β-actin to monitor for protein loading, as indicated at the right of each immunoblot. (A) The immunoblot of a representative time course of p73 expression is shown. (B) Two independent immunoblots from 2 different CLL samples were examined by densitometry using NIH Image software. The value before CD40-ligation (before) was subtracted from all time points as background value and set to 0%. The maximum value on day 3 after CD40-ligation was set to 100%. All other values were calculated relative to the maximum value [(intensity on day x / maximum intensity) × 100]. The graph shows mean values plus or minus the standard deviation of signal intensity on the y-axis relative to the time after CD154 treatment (eg, day 0 [D0], day 1 [D1], or day 3 [D3]), as indicated on the x-axis. (C) CLL cells were classified as having functional (p53 +) or nonfunctional p53 (p53-) by DNA sequencing of the p53 gene and/or by the functional assay of their response to γ-irradiation. Two patient samples each of CLL cells that were p53+ (samples from patients 1 and 2) or p53-(samples from patients 3 and 4) were analyzed 24 hours after treatment with HeLa cells (-) or HeLa-CD154 cells (+), as indicated at the top of each lane of the immunoblots.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/10/10.1182_blood-2006-04-017749/4/m_zh80220603580002.jpeg?Expires=1767790788&Signature=4kSo55OXGM~4Urj6Ms9aYuhvW0aPIa5mLk3fgyPh~3VIRkTmzPCh1lnIi4yvZ~6x70wgwD2oU0R2apM-lLdyQug8DKXgxcVpP~W2NOfQzqAXjaRaC70dNfmdmYkGfGooyzUGThPDSYbpXfeOfJEG505xBXF1ktpM5izfXSZWSaDeW8CgHYarYzttR0k9Vm9TtI8lYr8ZOuagUljFkSRlFhthWqWe3P3dWC6jTVnYPnQT0cV23W2v5MnWqfll6XZWPD1sa8quEfH6-fsfECaPS~lQxtQQvbisYbquBUNIYHej3B7G34QIGVYTfAuQA~3jXU6wcmk7CwpTFBgPSghkQw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)