Abstract
BACKGROUND: The Clot Formation and Lysis (CloFAL) global assay is a turbidimetric plasma assay utilizing coagluation activation by lipidated tissue factor and fibrinolytic enhancement by tissue plasminogen activator, and has recently been shown to be sensitive for factor VIII (FVIII) deficiency (Goldenberg et al., Thromb Res 2005; Goldenberg et al., Haemophilia 2006, in press). Deficiency of factor IX (FIX) is often clinically less severe than that of FVIII. We sought to evaluate the analytical sensitivity of the CloFAL assay for FIX deficiency, both in vitro and ex vivo.
METHODS: The influence of FIX activity upon the CloFAL assay was examined in vitro in FIX-deficient plasma by mixing study with pooled normal plasma, addition of plasma-derived FIX concentrate, and repletion with recombinant FIX. The analytical sensitivity of the CloFAL assay for FIX deficiency was then studied clinically via comparison of healthy individuals to FIX-deficient adults (n=25) and children (n=19), of whom greater than 1/3 had mild hemophilia B (FIX activity >/= 5.0 U/dL). FIX-deficient subjects had not received exogenous FIX within the prior 96 hours and had no evidence of active hepatitis. Healthy adult and pediatric groups (n=25 each) were without chronic illness or family history of bleeding or thrombosis. None of the study subjects were taking medications that affect hemostasis or had recent active bleeding or acute infection.
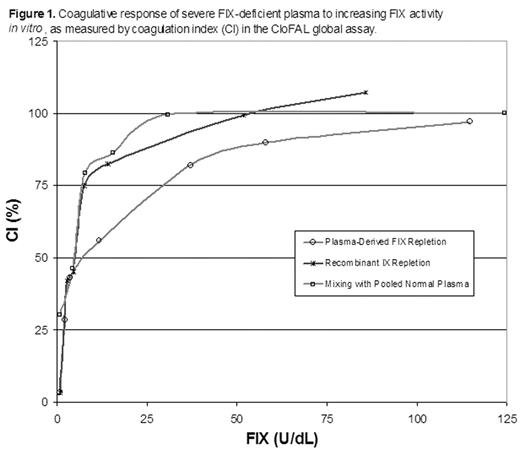
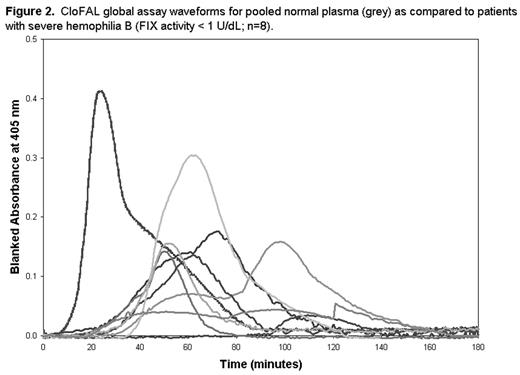
RESULTS: Alteration of FIX activity in vitro exerted considerable influence upon the CloFAL assay (Figure 1). Ex vivo, the CloFAL assay coagulation index (CI), a measure of the area under the clotting curve, was significantly decreased in FIX-deficient versus healthy subjects among adults and children alike (median CI, adults: 10% vs. 94%, respectively; median CI, children: 10% vs. 63%, respectively; P < 0.0001 for each), and correlated significantly with FIX activity in both age groups (r = 0.80 and r = 0.77; P < 0.0001 for each). In addition, the CloFAL assay fibrinolytic index (FI) was increased in FIX-deficient versus healthy subjects (median FI, adults: 228% vs. 109%, respectively, p<0.0001; median FI, children: 276% vs. 202%, respectively; p=0.7), although this difference was not statistically significant in children. Interestingly, severe hemophilia B patients (n=8; FIX activity < 1.0 U/dL) showed considerable heterogeneity in CloFAL assay waveforms (Figure 2). Nevertheless, the assay uniformly discriminated these patients from healthy controls.
CONLUSION: This work demonstrates that the CloFAL global assay, which is highly influenced by FVIII activity, is also analytically sensitive to FIX deficiency.
Coagulative response of severe FIX-deficient plasma to increasing FIX activity in vitro, as measured by coagulation Index (CI) in the CloFAL global assay.
Coagulative response of severe FIX-deficient plasma to increasing FIX activity in vitro, as measured by coagulation Index (CI) in the CloFAL global assay.
CloFAL global assay waveforms for pooled normal plasma (grey) as compared to patients with severe hemophilia B (FIX activity < 1 U/dL; n=8).
CloFAL global assay waveforms for pooled normal plasma (grey) as compared to patients with severe hemophilia B (FIX activity < 1 U/dL; n=8).
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author