Abstract
Sickle red blood cells (SS RBC) are abnormally adhesive to both endothelial cells (ECs) and components of the extracellular matrix (ECM). Epinephrine (epi) has been shown to elevate cAMP in SS RBC and increase adhesion of SS RBC to ECs in a protein kinase A-dependent manner. In vitro and in vivo studies performed in our lab have led to the hypothesis that adrenergic stimuli such as epi may initiate or exacerbate vaso-occlusion and thus contribute to the association of vaso-occlusive events with physiologic stress. We are conducting a prospective, dose-escalation pilot clinical study to investigate whether in vivo administration of one dose of propranolol either down-regulates baseline SS RBC adhesion in vitro or prevents its upregulation by epi. In addition, this study will provide additional safety data regarding the use of propranolol in normotensive patients with sickle cell disease (SCD).
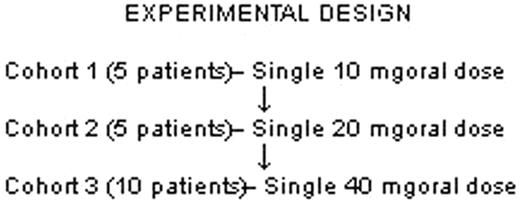
To date, we have completed the first two dose cohorts. 11 subjects (9 SS and 1 Sβ° thalassemia; 7 females, 3 males) have participated. No severe adverse events were noted. Cohorts 1 and 2 had mean pre-propranolol blood pressure (BP) of 116 (5.9 SD)/ 60.4 (3.98 SD) and 106.8 (4.68 SD)/ 58 (3.9 SD), respectively; this difference was not statistically significant. Minimal and asymptomatic changes in BP were noted in both cohorts after drug administration, with biphasic systolic and diastolic BP nadirs at 45 and 240 minutes. No clinically significant changes in heart rate were observed. Adhesion studies were performed using a graduated height flow chamber on the day of RBC collection. RBC adhesion to ECs was studied before and after epi stimulation and was measured at sheer stresses ranging from 1 to 3 dyne/cm2. Baseline adhesion measurements were validated by comparing percent (%) adhesion assayed at 2 different times within 7 days—at screening and before propranolol dose on the study drug day. We observed no significant difference in adhesion at the 2 different time points without propranolol. Comparison of % adhesion of epi-stimulated RBC to ECs before and 1 hour after propranolol showed that propranolol given in vivo significantly inhibited both non-stimulated and epi-stimulated SS RBC adhesion (p=0.04 and p=0.001, respectively). Lastly, comparison of SS RBC adhesion at both drug doses confirmed the drug-related inhibition of adhesion (p<0.004). We conclude that propranolol administered in vivo decreases SS RBC baseline adhesion to ECs and substantially abrogates epi-stimulated adhesion to ECs, as measured in vitro. Although we have thus far studied only a small number of patients and low propranolol doses, we expect to confirm these results with the 3rd cohort, in which a higher dose of propranolol will be used. If our findings continue to show that propranolol can decrease both SS RBC baseline and epi-stimulated adhesion to ECs, study of propranolol on a larger scale would be warranted in order to ascertain its safety and efficacy as an anti-adhesive therapy in SCD.
Disclosures: Discussion of use of propranolol for modification of cell adhesion in Sickle Cell Disease, used under an FDA waiver of IND.
Author notes
Corresponding author