Abstract
Pulmonary arterial hypertension (PAH) in sickle cell disease (SCD) is associated with chronic hemolysis and nitric oxide (NO) consumption and characterized by abnormal vascular tone, vascular proliferation and thrombosis. In previous studies, we demonstrated that increased platelet activation is linked to the severity of PAH in SCD. In addition, we reported that patients with SCD and secondary PAH have decreased platelet activation when given sildenafil, a finding that highlights the role of nitric oxide inhibition by hemolysis in this pathological mechanism. Since the vascular homeostasis is altered by the release of hemoglobin in plasma, we hypothesized that platelets of patients with PAH may show an enhanced degree of reactivity to platelet agonists released during hemolysis.
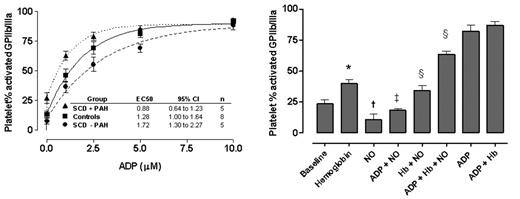
Whole-blood samples were used to determine the in vivo baseline and stimulated platelet activation from SCD patients with PAH, without PAH during steady state (not in pain crisis) and healthy controls. PAH was defined by a tricuspid regurgitant jet velocity ≥ 2.5 m/sec by Doppler echocardiography. Upon withdrawal, citrated venous whole blood was mixed gently with either phosphate-buffered saline (PBS) or escalating doses (1 – 10 μM final concentration) of adenosine diphosphate (ADP), a platelet agonist present in red blood cells. Platelet activation was measured by the flow cytometry detection of activated fibrinogen receptors (activated GPIIb/IIIa) and surface expression of P-selectin. Patients with SCD and PAH reached high degrees of activation with significantly lower concentrations of ADP (EC50 0.88 μM, 95% confidence interval 0.64 to 1.23, n=5) compared to controls (1.3 μM, 1.00 to 1.64, p < 0.01, n=8), and particularly to patients without PAH (1.7μM, 1.31 to 2.27, n=5)(Figure below, left panel). Similar results were observed in parallel experiments with thrombin-receptor activating peptide, another strong platelet agonist.
In separate experiments on platelets from healthy subjects, the addition of cell-free plasma hemoglobin induced platelet activation in dose-dependent fashion (p<0.05), supporting intravascular hemolysis as a mechanism of platelet activation in SCD (Figure below, right panel). Furthermore, cell-free hemoglobin blocked the ex vivo platelet inhibitory effects of an exogenous NO donor, MAHMANONOate (p<0.05), consistent with NO scavenging by cell-free hemoglobin.
These findings suggest that platelets from patients with SCD and PAH are unusually sensitive to activation. Our data also suggests that most of the variability in platelet hyperreactivity observed by other groups in SCD is primarily associated with PAH. Our data support a role for intravascular hemolysis and NO scavenging by cell-free hemoglobin in platelet hyperreactivity. Additional research is needed to define this mechanistic pathway in more detail.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author