Abstract
BACKGROUND: We have demonstrated the green tea extract epigallocatechin-3-gallate (EGCG) has anticancer activity in primary CLL B-cells (Lee, Blood 2004). After dissemination of our in vitro findings by the lay press, many patients with CLL and other low grade non-Hodgkin lymphomas (NHL) began using over the counter green tea extracts as an alternative treatment strategy. We recently reported a case series of 3 patients with CLL and 1 patient with follicular lymphoma who appeared to derive objective clinical benefit from such treatment (Shanafelt, Leukemia Research 2006). Based on these findings EGCG has entered clinical testing for treatment of CLL at Mayo Clinic. Here we explore the in vitro antitumor activity of EGCG against other types of non-Hodgkin lymphoma.
METHODS: Five established human B-cell lymphoma cell lines (HT, DOHH2, KARPAS, Ramos, RL) and primary lymphoma cells from 7 patients with various B-cell NHL sub-types [DLBC, FL, SMZ (2), MCL, SLL(2)] were used to evaluate the in vitro sensitivity of human lymphoma cells to EGCG. Freshly isolated primary lymphoma cells harvested in suspension from lymph nodes/spleen were obtained from patients with NHL who provided written informed consent. All patients were untreated at the time of biopsy. Lymphoma cell lines and primary lymphoma cells (n=7) were cultured with increasing doses of purified EGCG (3.12–50 ug/ml) for 24–72 hrs. Viability was assessed by using annexin/PI staining by FACS analysis.
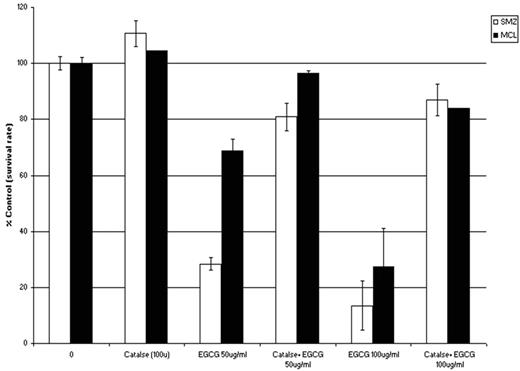
RESULTS: EGCG-induced dose dependent cell death in both established human B-cell lymphoma cell lines (average LD50 at 24 hrs between 25–50 ug/mL) and primary NHL cells (average LD50 at 24 hrs between 25–50ug/mL). In contrast, EGCG had minimal effect on purified normal B-cells (n=3) at the highest doses tested (50 ug/mL). By immunoblotting, EGCG-induced death in primary cells and cell lines was associated with PARP cleavage, suggesting the agent induced apoptotic cell death. Despite this finding, EGCG had no effect on levels of MCL-1, XIAP, or Bcl-1 by either immunoblot or FACS analysis. Based on reports that EGCG induces cell death in some cancer cell types through generation of oxidative stress (Furukawa, 2003; Nakazato, 2005), we explored this mechanism in lymphoma cells. To determine whether reactive oxygen species (ROS) generation was necessary for EGCG-induced cell death, lymphoma cell lines were cultured with or without catalase (which catalyzes the conversion of hydrogen peroxide to water and oxygen) for 30 min prior to subsequent 24 hr EGCG exposure (50 and 100 mg/ml). Pre-treatment with catalase (100 U) provided dramatic protection against cell death in both primary NHL cells and NHL cell lines suggesting that EGCG-induced cell death in lymphoma cells is dependent on ROS generation (Fig. 1 shows an example for a patient with mantle cell lymphoma and a patient with splenic marginal zone lymphoma).
CONCLUSION: EGCG has in vitro anti-tumor activity against a variety of B-cell NHLs. Given its known favorable toxicity profile in vivo, EGCG is an attractive agent for clinical testing in patients with indolent NHL who otherwise are currently being managed with observation.
Disclosures: Mitusi Norin.
Author notes
Corresponding author