Abstract
Lymphomatous meningitis (LM) occurs in approximately 7–15% of patients with lymphoma and carries an extremely poor prognosis (
Chamberlain et al. CNS Drugs 1998;10:25
; Chowdary & Chamberlain J Natl Compr Canc Netw 2005;3:693
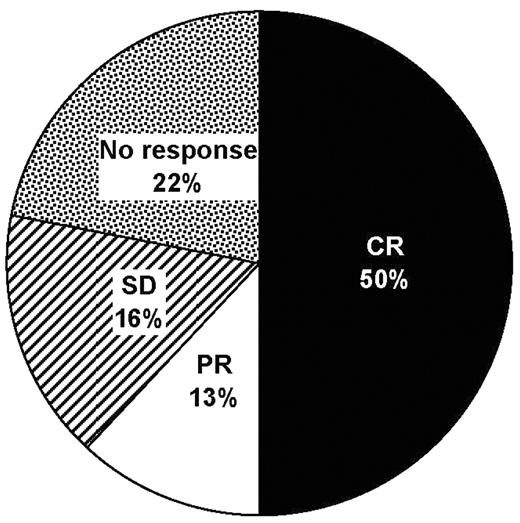
). Intrathecal (IT) chemotherapy with standard agents (cytarabine, methotrexate and thiotepa) is limited by the need for multiple injections per week via lumbar puncture or an Ommaya reservoir. Liposomal cytarabine (DepoCyte®) has an extended half-life in cerebrospinal fluid that permits fortnightly administration, improving convenience and reducing the potential for injection-related trauma and infections. Thirty-two Spanish patients (median age 43.5 years [range 19–78]; 22 male) with NHL received IT liposomal cytarabine for the treatment of LM between 2004 and 2006 at 21 treatment centers. Half of the patients had diffuse large B-cell lymphoma (DLBCL; n = 16); the remainder had Burkitt’s lymphoma (n = 4), T-cell NHL (n = 3), mucosa-associated lymphoid tissue lymphoma (n = 3), lymphoblastic lymphoma (n = 2), follicular lymphoma (n = 2) or primary CNS lymphoma (n = 1). A full histological diagnosis was not available for 1 patient. The dosage of liposomal cytarabine was 50 mg per cycle, with a median of 4 cycles (range 1–10). All patients received oral dexamethasone (4 mg 2–4 × daily for 4–7 days per cycle) as prophylaxis for chemical arachnoiditis. Neurological and cytological responses were obtained in 20 (62%; 16 complete responses, 4 partial responses; Figure 1) and 25 (78%) patients, respectively. Neurological progression was subsequently reported in 23 (72%) patients, with a median time to progression of 45 days (range 7–570). Twelve patients were still alive at the time of reporting, including 5 of 16 patients with DLBCL and 2 of 3 patients with T-cell NHL. Eighteen patients reported no adverse effects from treatment. The most commonly reported adverse effects were headache (n = 11), nausea (n = 4) and vomiting (n = 4). Data from this case series show that IT liposomal cytarabine is effective and well tolerated in the treatment of LM; the less intensive administration schedule of the agent may offer additional benefits to patients and their carers during the final months of life.Disclosure: No relevant conflicts of interest to declare.
Author notes
*
Corresponding author
2006, The American Society of Hematology
2006