Abstract
Background: Forodesine is a potent, rationally designed purine nucleoside phosphorylase (PNP) inhibitor that is orally bioavailable. IV forodesine has shown clinical activity in patients with cutaneous T-cell lymphoma (CTCL). The objective of this phase I/II study was to evaluate the safety, PK profile, and efficacy of oral forodesine in patients with refractory CTCL.
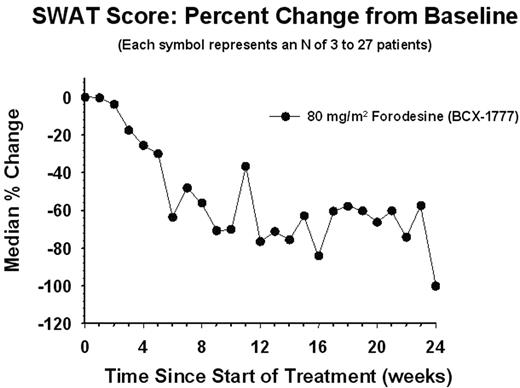
Methods: An open-label dose-escalation study of fordesine, 40 to 320 mg/m2 qd for 4 weeks, was undertaken to evaluate the safety and PK profile of oral forodesine, followed by an investigation of the expansion of the optimal biologic dose (dose with maximum PNP inhibition and elevation of plasma deoxyguanosine levels) to assess efficacy. Previously treated, refractory CTCL patients with stage IB or greater disease were eligible for participation. The primary end point was objective response (OR) = complete response [CR] plus partial response [PR], as measured by the severity-weighted assessment tool (SWAT; see Figure) and physicians’ global assessment (PGA)
Results: Overall, 37 patients were treated, including 14 patients in the dose-escalation portion of the study. No dose-limiting toxicities were observed with target doses of ≤320 mg/m2; thus, a maximum tolerated dose was never defined. Based on PK/PD results, an 80 mg/m2 qd dose was identified as the optimal biologic dose, and this ongoing study was expanded to enroll a total of 28 patients (median age, 64 yr; range, 28–81 yr). The OR rate was 53.6% (15/28 patients; 2 [7.1%] with a CR and 13 [46.4%] with a PR). The proportion of patients with stage IIB or greater disease was 19/28 (67.9%). Response in this population was seen in 10 (52.6%) of the 19 patients (1 [5.3%] with a CR and 9 [47.4%] with a PR). The percent change in SWAT score from baseline as a function of time demonstrated a progressive decrease over a 24-week period. To-date, 12 (42.9%) of 28 patients have completed ≥4 months of treatment. Safety data were recorded for all 37 patients treated with forodesine. The most common adverse events classified as grade 2 or less, without regard to causality, were nausea (30%), dizziness (22%), pruritus (22%), fatigue (19%), headache (19%), peripheral edema (19%), and pyrexia (16%). The only adverse event classified as grade 3 or greater, without regard to causality, and occurring in at least two patients, was lymphopenia, which was observed in two patients (5%).
Conclusions: Oral forodesine is effective in the treatment of refractory CTCL patients and has an encouraging safety profile.
SWAT SCORE: Percent Change from Baseline
Disclosures: Advisory Board member.; Clinical trials.; Honorarium from Advisory Board.
Author notes
Corresponding author