Abstract
Purpose: Bendamustine is a new anti-lymphoma agent with promising activity. Based on a preceeding phase I study the current trial explored Bendamustine in combination with Mitoxantrone and Rituximab (BMR) in patients with relapsed or refractory indolent lymphomas.
Patients and Methods: Patients with relapsed or refractory symptomatic stage III/IV indolent lymphomas with or without prior treatment with Rituximab were eligible. Therapy consisted of Bendamustine 90 mg/m2 days 1+2, Mitoxantrone 10 mg/m2 day 1, Rituximab 375 mg/m2 day 8. Treatment was repeated on day 29 for a total of 4 cycles.
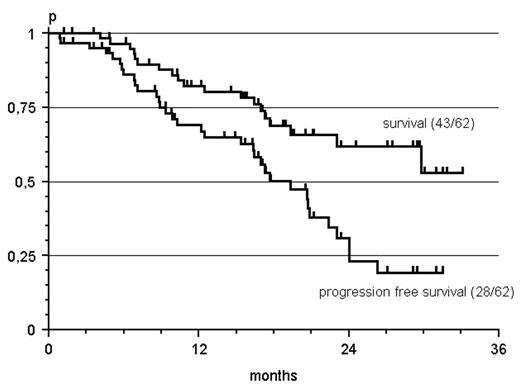
Results: Between 04/03 and 07/04 62 patients were recruited from 24 participating institutions, 40% of whom had received prior Rituximab therapy. Median age was 67 years (40–83). Lymphoma subtypes were 30 follicular (FL), 18 mantle cell (MCL), 4 B-CLL with plasmacytic differentiation, 3 lymphoplasmacytic (LPL), 3 marginal zone (MZL), 2 diffuse large B-cell (DLBCL), 1 high grade lymphoma not otherwise specified and 1 hairy cell leukemia. The overall response rate (ORR) was 88% with 36% CR and 53% PR. ORR in Rituximab pretreated patients was 75% (38% CR, 38% PR). After a median observation time of 17 months (1 – 33), the estimated median progression free survival is 19 months. Treatment related toxicities of grade 3/4 comprized a reversible myelosuppression (10 % anemia, 78 % leukocytopenia, 46 % granulocytopenia, 16 % thrombocytopenia). However, unexpected hospitalisations were necessary after 10 of 231 cycles (4%) only.
Conclusion: BMR is a very effective new outpatient immuno-chemotherapy with low toxicity for patients with relapsed/refractory indolent lymphoma.
Response rates
| . | All patients . | FL . | MCL . | pretreated with Rituximab . |
|---|---|---|---|---|
| n | 62 | 30 | 18 | 25 |
| end of therapy without staging | 3 | 3 | 0 | 1 |
| evaluable patients | 59 | 27 | 18 | 24 |
| CR | 21 (36%) | 13 (48%) | 6 (33%) | 9 (38%) |
| PR | 31 (53%) | 12 (44%) | 8 (44%) | 9 (38%) |
| MR | 1 (2%) | 0 (0%) | 1 (6%) | 1 (4%) |
| SD | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| PD | 6 (10%) | 2 (7%) | 3 (17%) | 5 (21%) |
| EX | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| CR+PR | 52 (88%) | 25 (93%) | 14 (78%) | 18 (75%) |
| . | All patients . | FL . | MCL . | pretreated with Rituximab . |
|---|---|---|---|---|
| n | 62 | 30 | 18 | 25 |
| end of therapy without staging | 3 | 3 | 0 | 1 |
| evaluable patients | 59 | 27 | 18 | 24 |
| CR | 21 (36%) | 13 (48%) | 6 (33%) | 9 (38%) |
| PR | 31 (53%) | 12 (44%) | 8 (44%) | 9 (38%) |
| MR | 1 (2%) | 0 (0%) | 1 (6%) | 1 (4%) |
| SD | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| PD | 6 (10%) | 2 (7%) | 3 (17%) | 5 (21%) |
| EX | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| CR+PR | 52 (88%) | 25 (93%) | 14 (78%) | 18 (75%) |
Disclosures: Restricted research grant for the conduction of the study from Hoffmann-La Roche and Ribosepharm.; Hoffmann-La Roche and Ribosepharm.
Author notes
Corresponding author