Abstract
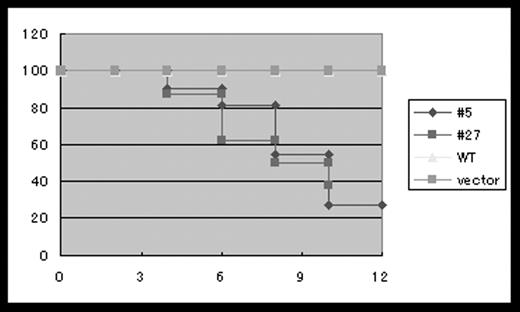
To understand myelodysplastic syndromes (MDS) at the molecular level, we used a mouse bone marrow transplant (BMT) model. Several leukemogenic fusion proteins were demonstrated to induce leukemia in mouse BMT models. However, analysis of molecular basis of MDS has been hampered by the lack of mouse MDS models. Mutations of a transcription factor AML-1/Runx-1 were identified in 15–40% of MDS-RAEB and MDS/AML and 5–10% of de novo AML. To test transforming abilities of the mutant AML-1, we transduced AML-1 with different mutations (#5 and #27) into mouse bone marrow cells using our highly efficient retrovirus-mediated gene transfer, transplanted the transduced cells to irradiated mice, and found that most mice developed leukemia within 4–13 months after the transplant (table1 and figure1). The leukemic mice exhibited marked hepato-splenomegaly, and morphological abnormalities of myeloid and erythroid lineages were frequently observed. Some mice developed to leukemia after a long latency with MDS-like symptoms, either with pancytopenia or leukocytosis with anemia, thus mimicking the human disease. To clarify the differences between the leukemic mice with early onsets and those that developed leukemia after a long period of MDS-like symptoms in the mutant AML-1-induced mouse diseases, the integration sites of the transduced retroviruses were investigated using a polymerase chain reaction (PCR)-based technique bubble PCR. Our preliminary results suggested that the integration sites of the retroviruses harboring the mutant AML-1 contribute to the early onset of leukemia. In conclusion, we have developed a useful mouse in vivo model of MDS/AML that should help understand the molecular basis of MDS and progression of MDS to leukemia.
penetrance and latency
| mutation number . | position . | form . | penetrance . | latency . |
|---|---|---|---|---|
| #5 | D171N | point mutation in RHD | 69.6 % | 4–13 months |
| #27 | S291fsX300 | truncation type | 100 % | 4–9 months |
| mutation number . | position . | form . | penetrance . | latency . |
|---|---|---|---|---|
| #5 | D171N | point mutation in RHD | 69.6 % | 4–13 months |
| #27 | S291fsX300 | truncation type | 100 % | 4–9 months |
Figure
Disclosures: TK serves as a consultant for R & D Systems, Rigel and Nihon Schering.; Chugai Pharmaceutical Co, Takeda Pharmaceutical Co.; Rigel, Kyowa Hakko Kogyo Co., Takeda Pharmaceutical Co.
Author notes
Corresponding author