Abstract
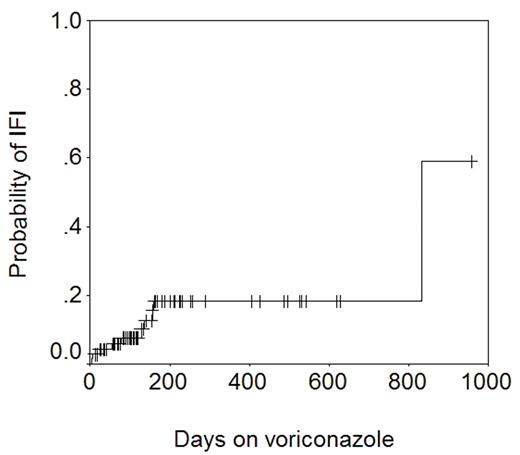
Voriconazole is a triazole anti-fungal agent with excellent activity against Aspergillus spp. We use liquid itraconazole 200 mg PO twice daily from day 0 to a month beyond discontinuation of all immunosuppression as standard anti-fungal prophylaxis after allogeneic HSCT in patients with no prior history of aspergillosis. This is changed to PO voriconazole 200 mg twice daily if corticosteroid therapy is started for GVHD. Voriconazole is continued even after steroid therapy is discontinued. Patients with a past history of aspergillosis get voriconazole from day 0. 71 allograft recipients who received voriconazole, and in whom complete clinical, microbiologic, and pharmacokinetic data were available were studied to determine the efficacy of voriconazole in preventing invasive fungal infections (IFI). 17 patients had not received itraconazole previously. The remainder had received itraconazole for 1–161 days (median 14). The length of voriconazole therapy was 6–956 days (median 133). The total number of patient-days on voriconazole was 13805 (~38 years). A total of 10 IFIs were seen in patients on voriconazole: Candida glabrata (n=5), Candida krusei (n=1), Cunninghamella (n=1), Rhizopus (n=2), and Mucor (n=1). The figure below shows the actuarial probability of IFI − 18% at 1 year.
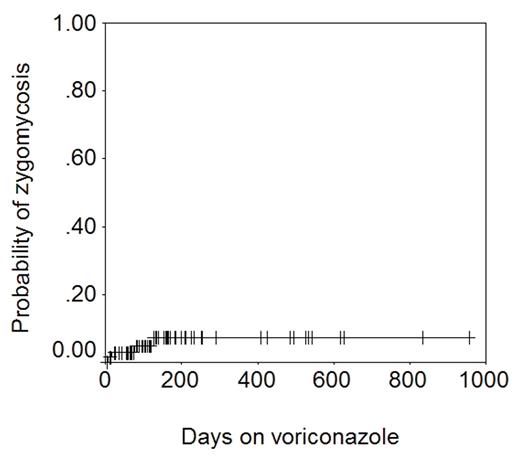
It is noteworthy that while 4 cases of zygomycosis were seen, no case of Aspergillus infection was seen. The figure below shows the actuarial probability of zygomycosis − 7% at 1 year.
Zygomycetes are generally not susceptible to voriconazole, and thus breakthrough infections are not surprising. However, C. glabrata and C. krusei are often susceptible to voriconazole with MICs of <2 μ g/mL (
Disclosures: Voriconazole for prophylaxis of invasive fungal infections.
Author notes
Corresponding author