Abstract
Introduction and aim of study. Cyclosporin A (CsA) is commonly used as prophylaxis against Graft versus Host disease (GVHD) after allogeneic haematopoietic stem cell transplantation (SCT). Therapeutic drug monitoring is necessary because of its unpredictable absorption and narrow therapeutic window. Usually dose adjustments are based on trough levels, although these correlate poorly with CsA systemic exposure in adults. The aim of this study was to investigate intravenous and oral CsA pharmacokinetics in children after SCT and to develop a limited sampling strategy in order to determine CsA systemic exposure.
Methods. Pharmacokinetics was investigated in children, aged 1.8 to 16.1 yrs, 2 to 43 days after stem cell infusion (median: 25 days), after intravenous or oral administration of CsA in, respectively, 9 and 8 children. Parameter estimation was performed using nonlinear mixed effect modeling as implemented in the NONMEM program.
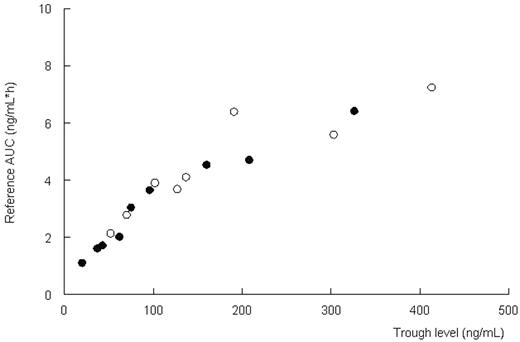
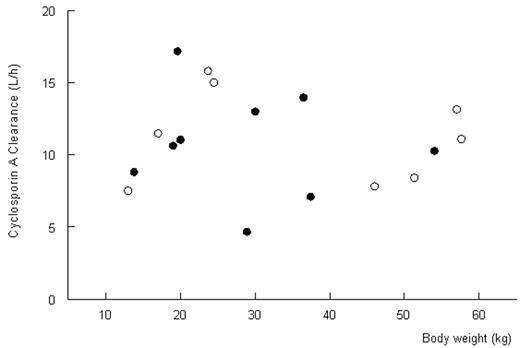
Results and conclusions. Pharmacokinetics were described adequately with a two-compartment model with lag time (population estimates: Cl=10.5 l/h; Vc=15.5 L; Vp=60.6L; t ½ absorption=1.01 h, Tlag=0.6 h). Combination of Cthrough and a Bayesian fitting procedure with the pharmacokinetic model can adequately estimate the systemic exposure to CsA (r2=0.90, Figure 1). The estimation of actual AUC improves when more concentration time-points are used. No relation between body weight and clearance was found (Figure 2). Based on these data, CsA should not be dosed per kilogram body weight. A regimen with a fixed dose for all, adjusted on through levels in combination with the described pharmacokinetic model, may be more appropriate. (open circles: oral administration; closed circles: intravenous administration)
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author