Abstract
OBJECTIVE: Mismatched allogeneic hematopoietic cell transplantation (alloHCT) carries a high risk of life-threatening graft-versus-host disease (GVHD) due to activation of donor T cells by antigens present on host cells. Removal of donor mature T cells can prevent GVHD but leads to an increased incidence of opportunistic infections and disease relapse. This study aims to selectively deplete host-reactive donor T cells responsible for GVHD while preserving T cells with anti-tumor and anti-viral effects.
METHODS: We utilized a photosensitizer, 4,5-dibromorhodamine-methyl ester (TH9402, Celmed Biosciences Inc., Saint-Laurent, Canada), in an ex vivo photodynamic purging (PDP) process to specifically eradicate host-reactive T cells. Donor T cells with anti-host specificity were identified in a unidirectional mixed lymphocyte culture (MLC) where they were activated and became proliferating. TH9402 is taken up by all cells and extruded out of the cell by P-glycoprotein (Pgp) in non-activated cells.
However, due to inactivation of Pgp in activated T cells, TH9402 is retained in the mitochondria. Upon exposure to 514 nm light in the Theralux™ device (Celmed), it becomes extremely cytotoxic resulting in cell death. In this study, after treatment with various concentrations of TH9402, the cells were exposed to light for the elimination of alloreactive T cells. The efficiency of allodepletion was assessed by Granzyme B (GrB) assay. T-cell proliferation assays were used to demonstrate the preservation of anti-tumor and anti-viral effects. Finally, the skin explant assay, an in vitro model of GVHD, was utilized to examine the efficacy of the PDP treatment in the removal of alloreactive T cells responsible for GVHD. The parameters of the PDP treatment were optimized for use in subsequent clinical studies.
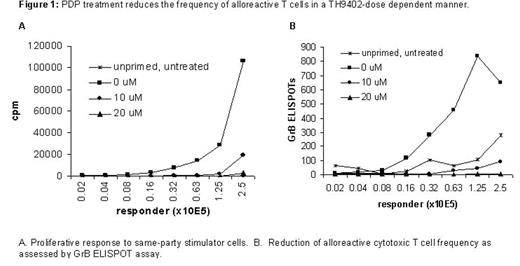
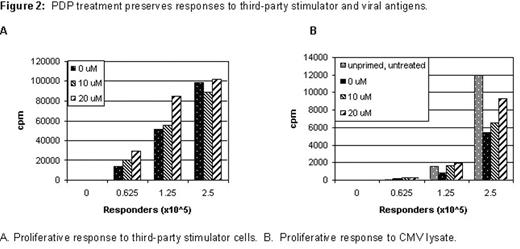
RESULTS: After 72-hour MLC, optimal proliferative response was obtained at a responder: stimulator ratio of 1:1. Activated T cells expressed high level of activation markers such as CD25 and CD69. After the PDP treatment with 20μM of TH9402, alloreactive T cells were consistently depleted by more than 2 logs (Figure 1). Moreover, the PDP treatment did not significantly affect anti-tumor and anti-viral effects as evidenced by responses to third-party stimulators (Figure 2A), cytomegalovirus (CMV) (Figure 2B) and Candida antigens. Most importantly, co-culture of recipient’s skin with PDP-treated cells showed a reduction of graft-versus-host reaction (GVHR) in a TH9402-dose dependent manner. The PDP treatment with 20μM of TH9402 completely abolished GVHR in a skin explant assay.
CONCLUSIONS: The PDP treatment can effectively remove donor T cells responsible for GVHD while preserve T cells with anti-tumor and anti-viral effects. These preclinical results provide a basis for initiating a clinical trial to assess the feasibility and efficacy of infusing PDP-treated donor T cells to alloHCT recipient in order to augment anti-tumor and anti-pathogen effects without causing GVHD.
PDP treatment reduceds the frequency of alloreactive T cells in a TH9402 does dependent manner.
PDP treatment reduceds the frequency of alloreactive T cells in a TH9402 does dependent manner.
PDP treatment preserves responses to third-party stimulator and viral antigens.
PDP treatment preserves responses to third-party stimulator and viral antigens.
Disclosures: Part of this work was performed under a collaborative research and development agreement between Duke University Medical Center and Celmed Biosciences Inc.
Author notes
Corresponding author