Abstract
Background: MM is an incurable disease with an anticipated overall survival ranging from months to decades. Novel therapies like bortezomib have activity in both the relapsed/refractory and up front settings. There are sparse data on whether novel therapies may overcome high risk features.
Methods: Patients with newly diagnosed high-risk myeloma (beta-2 microglobulin [B2M] >= 5.5., plasma cell labeling index [PCLI] >= 1, or deletion 13q) were eligible. Patients were treated with bortezomib 1.3 mg/m2 day 1, 4, 8, and 11 every 21 days for 8 cycles as induction. After induction, patients received bortezomib 1.3 mg/m2 every other week indefinitely. Patients relapsing on maintenance schedule resumed full induction schedule. Responses were defined by the EBMT criteria. The primary end-point was the response rate (90% power to detect a response rate of >=50% ).
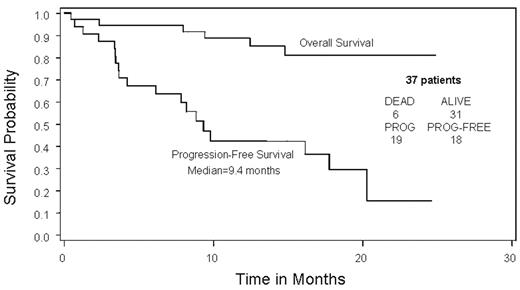
Results: Between March 15, 2004 and March 10, 2005, 44 patients enrolled. Among the 43 eligible patients, median age was 63; 51% were male. All had high risk disease: deletion 13q (6/41); PCLI >=1% (17/33); t(4:14) (4/27) and B2M >= 5.5 (34/43). Response data are available for 37 of the 43 eligible patients. The overall response rate was 49% (95% CI: 0.32, 0.66) (0 patients with CR, 1 VGPR, 15 PR, 2 MR, and 11 inevaluable). The response rate allowing for use of serum monoclonal protein levels when a measurable urine monoclonal protein was unavailable at follow-up was 59.5% (1 patient with CR, 1 VGPR, 18 PR, 2 MR, and 6 inevaluable). The median progression free survival is 9.4 months. Thirty-three percent of patients completed the 8 cycles of planned induction therapy and moved to maintenance. Reasons for discontinuing therapy have included progression or death (n=18), adverse events (n=6), and other (n=14). Only 12% of patients remain on active therapy: 0% of deletion 13q patients; 25% of t(4:14) and 12% each of high B2M and high PCLI patients. Of the 14 patients who entered the maintenance phase of treatment, 3 have progressed. Of these 3, 2 took re-induction, and neither responded. Median time to progression for those entering maintenance was12.4 months from the time of starting maintenance. The most common adverse events of grade 3 or higher included neutropenia (33%), diarrhea (31%), hyponatremia (21%), anemia (19%), thrombocytopenia (16%), fatigue (14%). Grade 1–2 sensory peripheral neuropathy occurred in 53% of patients, with only 2% having grade 3 sensory neuropathy. One patient had grade 3 peripheral neuropathy. One patient died after receiving 2 doses of protocol treatment due to heart block and asystole. Updated results on the full study population will be presented at the meeting.
Conclusions: In high risk patients, upfront bortezomib appears to result in comparable response rates to those reported for unselected cohorts of newly diagnosed myeloma patients. Continued follow-up of these patients will provide information about whether this will translate into better overall outcomes.
Disclosures: Bortezomib is not yet approved for first line use in multiple myeloma.
Author notes
Corresponding author