Abstract
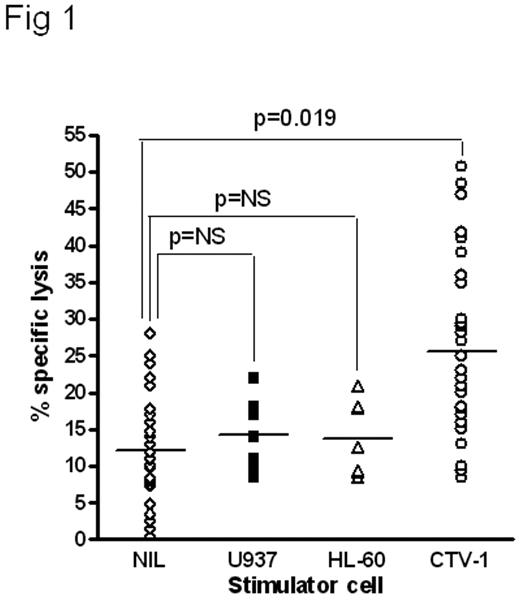
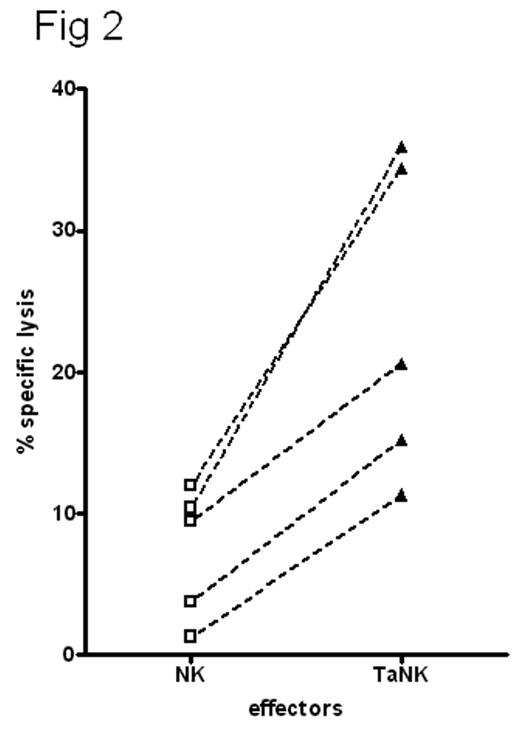
Pre-incubation of resting human NK cells with the leukemia cell CTV-1 primes NK cells to lyse NK-resistant cell lines, primary leukemias and solid tumors, even when HLA-matched; allogeneic or autologous. The primed NK cells remained non-responsive to HLA-C matched and mismatched normal peripheral blood or bone marrow mononuclear cells from multiple donors and did not affect in vitro colony forming unit assays. NK cells isolated from normal healthy donors and co-incubated with irradiated CTV-1 tumor cells synthesized CD69 within 60 min with maximal expression achieved within 6 hr. The tumor-activated NK cells (T-ANKs) lysed NK resistant RAJI and Daudi cells in a 4-hour assay (Fig 1). The degree of lysis was equivalent to that of matched lymphokine activated killer cells (LAK) after non-specific activation with IL-2. CTV-1 cells are HLA-C type 2 homozygous and express HLA-Bw4 alleles. They thus can ligate KIR2DL1 (CD158a) and KIR3DL1 (CD158e1) on NK cells. Co-cultures of HLA-matched or mis-matched NK with CTV-1 showed there was no significant difference in the generation of T-ANK activity in the presence or absence of KIR ligation. T-ANK cells from allogeneic donors were capable of lysis of primary AML cells of all FAB types, the relatively NK-resistant breast cancer cell line, MCF-7, and primary tumor cells isolated from resected tissue from patients with breast cancer and ascities from patients with ovarian cancer. Autologous T-ANK cells were generated from five AML patients in clinical remission; all of whom had low level or undetectable NK activity to their presentation disease. T-ANK cells from all five donors consistently induced significantly greater lysis of autologous AML blasts than the matched NK cells (p<0.05) (Fig 2). To investigate the tumor-restriction of allogeneic T-ANK cells we isolated NK cells from normal donors and either activated them with CTV-1 cells overnight or maintained them in media. These T-ANK cells were then compared with NK cells from the same donor with respect to lysis of normal autologous and allogeneic PBMC. Neither NK nor T-ANK cells lysed autologous PBMC nor did they lyse PBMC from HLA-C mismatched normal donors. To determine the likelihood of bone marrow suppression by T-ANK cells we established hematopoietic colony forming assays with bone marrow from 5 normal donors and added T-ANK from HLA-C mismatched donors at increasing ratios. CFU-GM, BFU-E and CFU-GEMM were not affected by co-incubation with HLA-mismatched T-ANK even at effector:target ratios of 20:1. We believe that this demonstrates a two stage process for NK activation; priming and triggering, the ligands for which are distinct. Tumor priming of NK primes cells capable of lysing a broad spectrum of NK-resistant tumors which may irrespective of KIR ligation which may represent an “off-the-shelf” immunotherapy product.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author