Abstract
Introduction: Hepatic siderosis, a result of chronic transfusion therapy, leads to hepatocellular injury and progression to chronic liver disease. Increases in serum transaminase levels are indicative of progressive liver damage. The effect of deferasirox (Exjade®, ICL670), a novel, once-daily oral chelator, on liver iron content and pathology in chronically transfused patients has been assessed in Phase II and III clinical studies. A sub-analysis of pooled data from the planned 4-year extension of two pivotal studies sought to correlate the long-term changes in serum ALT levels with those of serum ferritin in iron-overloaded patients during treatment with deferasirox.
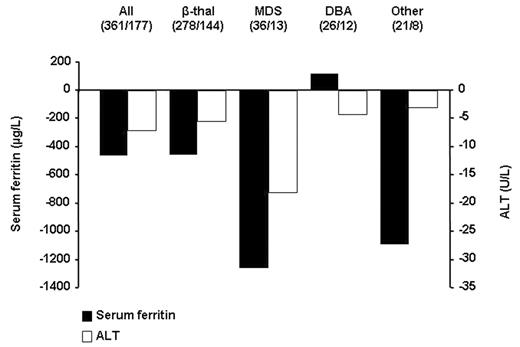
Methods: In these two clinical studies, 480 patients with β-thalassemia (β-thal), myelodysplastic syndromes (MDS), Diamond-Blackfan anemia (DBA), and other transfusion-dependent anemias received deferasirox at 5, 10, 20 or 30 mg/kg/day according to baseline liver iron concentrations for 12 months. In the extension phases, doses were titrated according to patient response, with dose escalation for patients who did not receive sufficient drug in the core study to reduce iron burden. Serum ferritin and ALT levels were assessed at baseline and monthly during the core and extension phases. For this analysis, serum ferritin and ALT levels were analyzed in the combined patient groups who received deferasirox 5 and 10 mg/kg/day, and in those who received 20 and 30 mg/kg/day.
Results: Throughout the entire period, deferasirox at 20 and 30 mg/kg/day resulted in a negative iron balance in transfused patients with secondary iron overload. Changes from baseline to week 128 (2.5 years) for serum ferritin and ALT levels are presented. The figure shows that deferasirox at 20 and 30 mg/kg/day decreased median serum ferritin. These decreases were mirrored by decreases in ALT, with a Spearman’s rank-correlation 0.67. Patient numbers shown are baseline/week 128. A number of patients had not reached week 128 of treatment at the time of data cut-off.
Decreases in ALT were also observed after dose escalation in the extension phase in patients originally assigned deferasirox 5 and 10 mg/kg/day. There were no consistent relevant changes in other biochemical liver parameters, such as serum aspartate transaminase, in this patient population.
Conclusions: With long-term follow-up of this large patient population, correlation between decreases in serum ferritin and ALT levels was high, indicating an association between iron burden and liver dysfunction, and further emphasizing the potential beneficial effect of deferasirox on hepatic function.
Disclosures: B Rabault is an employee of Novartis Pharma AG.; P Brissot - an expert member for Novartis in Exjade evaluation.; P Brissot - a centralized study, in our hands, of liver iron concentration in the tested population; H Cario - from Novartis in relation to the studies on deferasirox and for the establishment of diagnostic tools for the assessment of liver iron concentration; M Jeng - Has received research funding from Novartis Pharmaceuticals.; P Brissot - Novartis; M Jeng - Novartis Pharmaceuticals.; P Brissot - an expert member for Novartis in Exjade evaluation; M Jeng - Member of Speaker’s Bureau and Advisory Committee for Novartis Pharmaceuticals.
Author notes
Corresponding author