Abstract
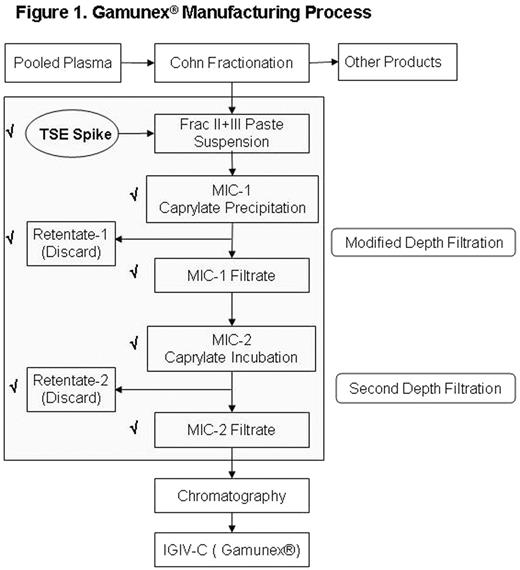
Prions are misfolded pathogenic proteins associated with the transmission of transmissible spongiform encephalopathies (TSEs). Although no TSE transmission through human plasma products has been documented to date, the risk of transmission exists. To provide assurance for product safety with respect to the risk of TSE transmission, this study evaluates the prion clearance potential of a modified intravenous immunoglobulin (Gamunex®) manufacturing process (Figure 1).
Gamunex® Manufacturing Process
In the process prior to modification, an intermediate material was subjected to cloth filtration. In the modified process, the cloth filtration is replaced with a depth filtration to improve pathogen removal capacity. Both processes were studied using validated scaled-down models, along with a spiked prion agent. The modified process reduced the prion level to below the limit of detection by Western blot assay. The animal bioassay for infectivity confirmed that the modified process has a marked improvement in prion clearance. This modified process has been implemented in actual Gamunex® manufacturing process. Gamunex® is the only liquid IGIV with FDA approved prion clearance data.
Disclosures: All authors are employed by Talecris Biotherapeutics.
Author notes
Corresponding author