Abstract
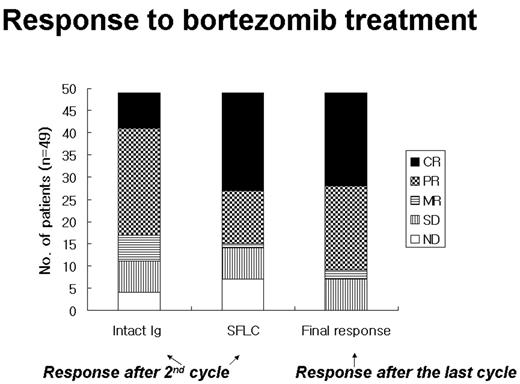
Bortezomib alone or in combination with chemotherapeutic agents produce a rapid disease control in patients with multiple myeloma (MM). However, laboratory factors predictive of outcome with bortezomib remain obscure. The aim of this study is to determine whether serum free light chain (SFLC) measurements could be a new sensitive test for the early detection of response to treatment with bortezomib and to perform an analysis of biochemical markers to determine their value in predicting response. Data from evaluable 49 patients receiving 2–7 cycles (median, 4) of bortezomib were analyzed. During the first and second cycles of bortezomib treatment, serial serum samples were prospectively collected for simultaneous measurement of SFLC, intact immunoglobulin (Ig) and biochemical markers such as lactic dehydrogenase (LDH), alkaline phosphatase (ALP), uric acid, calcium and phosphorus. SFLC and Ig were measured on day 0 and 12 each cycle and the biochemical markers on day 0, 2, 5, 9 and 12. Twenty-seven patients, 10, 1, 1 and 8 were IgG, IgA, IgM, IgD myelomas and light chain disease (LCD), respectively. Two patients did not secrete monoclonal protein. Patients received bortezomib alone (n=25) 1.0–1.3 mg/m2 for 3–4 week cycles along with various combinations including dexamethasone, thalidomide and/or doxorubicin (n=24). Forty of 49 patients (81.6%) showed an objective response (CR+PR) response upon completion of bortezomib treatment while 9 patients had <PR by EBMT criteria according to monoclonal Ig concentration. Thirty-two of 39 patients (82.1%) with intact Ig MM patients had an abnormal SFLC concentration kinetics after the second bortezomib treatment. All 8 patients with LCD and 2 patients with non-secretory MM showed elevated concentrations of one or both SFLC. In comparison to the intact Ig levels, SFLC concentrations fell more rapidly in response to bortezomib treatment and the pattern of initial SFLC response seems to be an early indication of tumor response or resistance (see the figure; the response after the second cycle was assessed by concentrations of intact Ig or SFLC, respectively). The increase of LDH levels from baseline between two groups during and upon completion of two cycles of therapy was statistically significant (P=0.001). The increase of UA levels from baseline exhibited a marginal significance (P=0.081). In addition, we observed significantly higher mean ALP elevation in the responder group compared with the non-responder group during the two cycles (P=0.027). The monitoring of SFLC provides a unique opportunity to follow the kinetics of tumor kill especially when the monoclonal Ig was not detected. In addition, changes in SFLC concentrations can be used as an early biomarker to assess a rapid response to bortezomib treatment. Biochemical marker assays showed that the response to bortezomib might be associated with tumor lysis and/or osteoblastic activation.
Response to bortezomib treatment
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author