Abstract
Background: Over the past decade new treatment options and drugs significantly altered the treatment paradigm and treatment guidelines for patients with MM. We reported in 2005 initial results from a treatment survey in GER (Freund ASH2005# 5158; Angermund, ASH2005, #5156). Bortezomib (Velcade®, Vel) at that time (6 months (m) after approval) was the 2nd most frequently (freq) used drug after dexamethasone (dex) in the approved indication (3rd line) and played a minor role in 2nd line therapy. The survey performed in 1st quarter 2006 (1.5 years (y) after the initial survey), used identical methods and questionnaires. These results allow for monitoring of changes in treatment patterns.
Methods: The method of this representative analysis was provided elsewhere (Freund ASH2005# 5158). The data presented here are a subset analysis based on 66 sites and 428 patients.
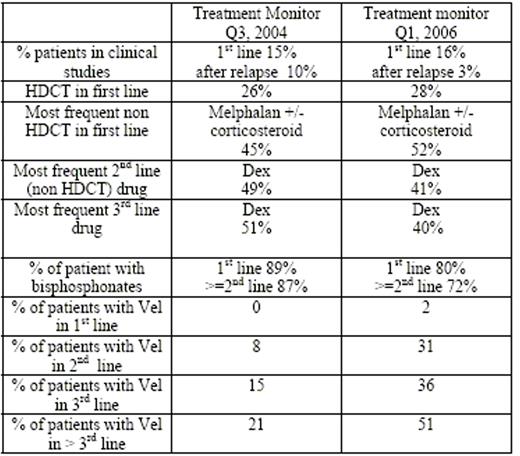
Results: 230 male and 195 female patients, 43% at the time of analysis for decision on primary therapy, 27% for secondary treatment and 30% for further treatment were included in this analysis. The centers participating were 11% university hospitals (UH), 38% non-university hospitals with specialized (SH) and 20% without specialized (NSH) haematology department, and 32% office-based haematologists (OBH). The main treatment in primary therapy did not change (table) whereas Vel gets more commonly used. The increase in Vel in all treatment lines is mainly due to SH (64 (2006) vs. 52 % (2004); OBH: 32 (2006) vs. 32% (2004); NSH: 0 (2006) vs. 2 % (2004); UH 4 (2006) vs 14% (2004)). In 2nd line Vel was the 2nd most prescribed drug (31%) (1st dex 41%; 2004: Vel 8% ranked 10th) mainly in UH/SH in patients below 60 y and after primary high dose chemotherapy (HDCT) (35% Vel vs. 28 % all) and with longer remission period (median 9 m Vel, median 7 m all). Within treatment ≥ 3rd line, Vel was the most freq used drug followed by dex (42% and 38% respectively). For patients currently in 1st remission Vel was most freq planned as next therapy (45%) followed by lenalidomide (len) (19%) and dex (12%). Vel was mostly used by SH following melphalan or VAD/VID treatment (multivariate analysis). Len and thalidomide (thal) (both drugs not approved in GER at that time) are rarely used in MM therapy (0% len vs. 10% thal in ≥ 2nd line).
Conclusion: New approved treatment options like Vel are quickly integrated into treatment behaviour in GER, mainly used in SH within approved indication, whereas not approved drugs like len or thal play only a minor role. 9 m after approval for 2nd line therapy, Vel increased uptake within ≥ 2nd line indicating this drug’s possibility to become a future treatment standard in pretreated patients. In order to detect the dynamic of change of treatment of MM, further follow up is planned.
Disclosures: Bortezomib in first line use.; R Angermund & H Pliskat employees of Ortho Biotech.; R Angermund & H Pliskat Ortho Biotech.; H Einsele, H Goldschmidt, C Straka Research funding.; M. Freund, M. Nowrousian, H. Einsele, H. Goldschmidt, W Berdel, W Poenisch, C Straka & W Knauf members of advisory board.
Author notes
Corresponding author