Abstract
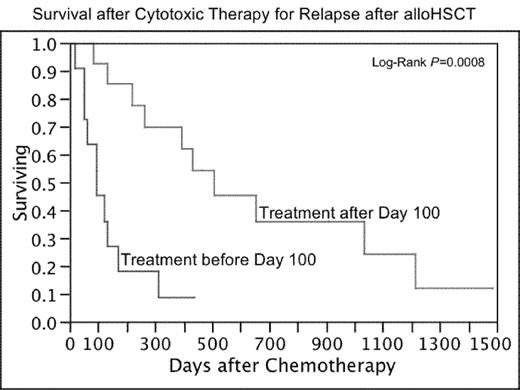
Relapse of hematologic malignancy is a major problem after allogeneic hematopoietic stem cell transplantation (alloHSCT), with limited treatment options of proven benefit other than donor lymphocyte infusions (DLI). Additionally, toxicity from treatment with cytotoxic chemotherapy after alloHSCT may differ from conventional treatments, and could include graft-versus-host disease, allograft failure, or increased risk of infection. We retrospectively reviewed treatment outcomes of 25 patients who received cytotoxic chemotherapy as treatment for relapsed hematologic malignancies after reduced-intensity, matched-sibling donor alloHSCT. Combination therapies included: EPOCH+/− rituximab (n=12 patients); “7&3” AML induction (ida/ara-c) (n=2); FLAG (n=2); asparaginase/6-MP/vincristine (n=1); R-ICE (n=1); MIME (n=1); BVP (n=1); and bortezomib/doxorubicin/dexamethasone (n=1). Single-agent therapies included: bortezomib (n=6); gemcitabine (n=4), vinorelbine (n=3); vincristine (n=1); methotrexate (n=1); and intrathecal methotrexate and/or cytarabine (n=5). A total of 133 cycles or doses were administered, of which 20 were supported with donor stem cell boosts, and another 9 were followed by nonmobilized DLI. There were 52 Grade 3 or higher toxicity episodes recorded, most commonly: neutropenia (10 episodes); infection with neutropenia (5 episodes); thrombocytopenia (6 episodes); and anemia (3 episodes). There were 12 episodes of CMV reactivation in 8 of 15 patients at risk, including 2 cases of pneumonitis and 1 case of colitis. GVHD flares were considered definitely chemotherapy-induced (2), possibly chemotherapy-induced (7), and unlikely chemotherapy-induced (2). Unusual events after treatment included culture-negative sepsis-like illness after bortezomib (n=2), secondary (11q23) AML of donor origin after one cycle of EPOCH (n=1) and diffuse alveolar hemorrhage after EPOCH (n=1). Seven patients achieved CR with therapy for post-transplant relapse, including three durable remissions. Four patients achieved PR and seven achieved disease stabilization. Median survival after starting cytotoxic therapy was 263 days. Timing of relapse appeared to be associated with survival, with median overall survival of 95 days for patients requiring therapy before Day 100 vs. 508 days for patients after Day 100 (p=0.0008). Treatment of relapse with cytotoxic chemotherapy after alloSCT is feasible and can result in durable remissions in a minority of patients. However, administration of cytotoxic therapy after alloHSCT requires monitoring and support for hematologic toxicities, GVHD and CMV reactivation; selected patients may benefit from prophylactic stem cell boosts or immune suppression.
Survival after Cytotoxic Therapy for Relapse after alloHSCT
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author