Abstract
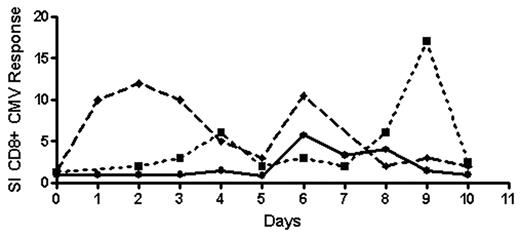
CMV reactivation after allogeneic stem cell transplantation remains a major cause of post-transplant morbidity but may be mitigated by strong memory T cell responses in the transplanted donor T cell repertoire. To induce CMV specific T cell responses in CMV seronegative donors and to boost CMV reactivity in seropositive stem cell donors we vaccinated donors and healthy volunteers with the ALVAC-pp65 live attenuated canarypox vaccine (sanofi pasteur, Lyon, France). A safety study in 4 normal donors confirmed that apart from variable local reactions, an accelerated vaccine schedule was well tolerated. We then studied a similar accelerated regimen giving 1.0 ml intramuscular ALVAC on days 0, 5, and 10 and measured CD4+ and CD8+ T-cell responses to a CMV pp65 peptide library using flow cytometry to measure the frequency of interferon-gamma producing cells. Blood was drawn for testing on days 0, 5, 10, 30, 60, and 90. Positive responses were defined as a two fold increase of interferon-gamma production compared to unstimulated lymphocytes. Four seropositive and eight seronegative individuals were studied. Of the 8 seronegative individuals, 4 were found to have pre-vaccine CD4+ and CD8+ responses to pp65 and were considered to be CMV-experienced individuals. Three of these 4 individuals had a CD4+ and CD8+ response exceeding baseline at some time between days 30 and 90. All 4 CMV-naïve individuals had responses to ALVAC-pp65 by day 30. Of the four seropositive individuals, 3 lacked detectable CD4+ and 2 lacked CD8+ T cell responses at baseline. The 2 subjects that lacked both CD4+ and CD8+ responses at baseline had positive responses with vaccination. To explore the possiblility that immediate early responses might be rapid and transient in CMV-exposed individuals, three donors (one seronegative) were monitored daily during the first 10 days of the vaccine series. All showed significant responses 1–4 days after second vaccination (see figure). Ten patients (8 with hematological malignancies) were recipients of HLA-matched stem cell transplants from vaccinated donors. Four reactivated CMV, two were recipients of T cell depleted transplants and no patient had CMV disease. These results show that ALVAC-pp65 is a well-tolerated vaccine with the potential to induce pp65 cellular immunity in CMV-naïve individuals and boost responses in CMV-experience individuals. However, the kinetics of the CMV response differs according to whether the T cell response exists at baseline but interestingly does not correlate with serostatus. To deliver a high frequency of pp65 specific T cells to transplant recipients, the optimum schedule for CMV exposed donors appears to be after second vaccination. In contrast, longer periods are required in CMV-naïve donors. Based on these findings, we will use appropriately-timed cell collections from ALVAC-pp65 vaccinated donors to explore whether CMV-exposed transplant recipients can be protected from CMV reactivation.
Disclosure: No relevant conflicts of interest to declare.
Author notes
Corresponding author