Abstract
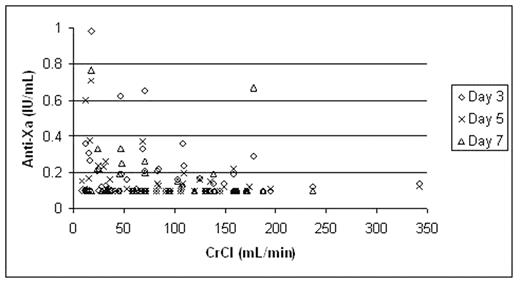
Low molecular weight heparin (LMWH) is predominantly eliminated by the kidneys. In patients with severe renal impairment, use of therapeutic dose LMWH may be associated with accumulation and a resultant bleeding risk. Tinzaparin may be less dependent on renal clearance due to its higher molecular mass and greater negative charge compared to other LMWHs. The objective of this prospective cohort study was to serially measure the anti-Xa anticoagulant effect of therapeutic dose tinzaparin over 5–7 days, used for the initial treatment of venous thromboembolism (VTE) in patients with varying degrees of renal insufficiency. We present the anti-Xa results from the first 78 patients enrolled in the study, correlated with renal function. In this study, consecutive in- and outpatients with objectively confirmed VTE requiring anticoagulation were enrolled and stratified into 4 groups based on the calculated Cockcroft-Gault creatinine clearance (CrCl): > 60 mL/min, 30–60 mL/min, ≤ 30 mL/min and hemodialysis-dependent. Tinzaparin 175 IU/kg was administered subcutaneously once daily for 5–7 days or until the INR ≥ 2.0 with warfarin therapy. Trough anti-Xa levels were measured prior to the 3rd, 5th and/or 7th tinzaparin doses. Patients with anti-Xa level > 0.5 IU/mL received tinzaparin dose adjustment using a standardized nomogram. Bleeding and recurrent VTE events were recorded. The relationship between anti-Xa levels and CrCl is shown in Figure 1. Based on our predefined anti-Xa threshold of 0.5 IU/mL, 5 of 78 patients (6.4%) required dose adjustment; 1 hemodialysis dependent patient, 2 patients with CrCl < 30 mL/min, 1 patient each with CrCl 30–60 and > 60 mL/min, respectively. None of these patients developed bleeding or recurrent VTE. Among all 78 patients, 1 hemodialysis-dependent patient developed a hematoma following a traumatic line insertion, and no patients developed recurrent VTE. In conclusion, in a cohort of 78 patients with differing degrees of renal function including patients requiring hemodialysis, use of therapeutic-dose tinzaparin for the initial treatment of VTE resulted in accumulation (defined by trough anti-Xa > 0.5 IU/mL) in 6% of patients. There appears to be a weak inverse relationship between renal function and trough anti-Xa levels, but does not result in clinically significant accumulation when tinzaparin is used for up to 7 days. Further evaluation of tinzaparin in patients with severe renal insufficiency is required.
Disclosures: Unrestricted educational grant from Leo Pharma used to purchase tinzaparin for this study.; Honoraria received from Leo Pharma for presentations.; Advisory board participant for Leo Pharma.
Author notes
Corresponding author