Abstract
Tandutinib (MLN518/CT53518) is a novel quinazoline-based inhibitor of the type III receptor tyrosine kinases: FMS-like tyrosine kinase 3 (FLT3), platelet-derived growth factor receptor (PDGFR), and KIT. Because of the correlation between FLT3 internal tandem duplication (ITD) mutations and poor prognosis in acute myelogenous leukemia (AML), we conducted a phase 1 trial of tandutinib in 40 patients with either AML or high-risk myelodysplastic syndrome (MDS). Tandutinib was given orally in doses ranging from 50 mg to 700 mg twice daily The principal dose-limiting toxicity (DLT) of tandutinib was reversible generalized muscular weakness, fatigue, or both, occurring at doses of 525 mg and 700 mg twice daily. Tandutinib's pharmacokinetics were characterized by slow elimination, with achievement of steady-state plasma concentrations requiring greater than 1 week of dosing. Western blotting showed that tandutinib inhibited phosphorylation of FLT3 in circulating leukemic blasts. Eight patients had FLT3-ITD mutations; 5 of these were evaluable for assessment of tandutinib's antileukemic effect. Two of the 5 patients, treated at 525 mg and 700 mg twice daily, showed evidence of antileukemic activity, with decreases in both peripheral and bone marrow blasts. Tandutinib at the MTD (525 mg twice daily) should be evaluated more extensively in patients with AML with FLT3-ITD mutations to better define its antileukemic activity.
Introduction
The success of BCR-ABL kinase inhibition by imatinib mesylate in the treatment of patients with chronic myelogenous leukemia1-5 has provided a stimulus to the development of other kinase inhibitors as potential therapies for hematologic malignancies. FMS-like tyrosine kinase 3 (FLT3) is a transmembrane protein that belongs to the type III receptor tyrosine kinase family. Other members of this family include platelet-derived growth factor receptor (α/β-PDGFR), KIT, and CSF1R. Activating mutations of FLT3 are found in 20% to 30% of patients with newly diagnosed acute myelogenous leukemia (AML), the majority of these taking the form of an internal tandem duplication (ITD) in the juxtamembrane region of the receptor.6-12 Activating point mutations in the kinase activation loop of the receptor also occur but with lower frequency (5%-10% of patients newly diagnosed).9-14 Both ITD and activation loop mutations appear to have a negative effect on prognosis: patients with these mutations relapse sooner following initial induction chemotherapy and have inferior survival compared with patients with only the wild-type receptor.6-8,10,12,14-17
Tandutinib is a piperazinyl quinazoline compound that resulted from screening of chemical libraries and subsequent optimization.18,19 In cell-based assays tandutinib inhibited FLT3, β-PDGFR, and KIT with IC50 values of 95 to 122 ng/mL but had no significant effect against a broad range of other kinases.18,19 In Ba/F3 cells expressing various FLT3-ITD mutants, tandutinib inhibited IL-3–independent growth and FLT3-ITD autophosphorylation with IC50 values of 6 to 17 ng/mL.19 Tandutinib also inhibited in vitro proliferation of human leukemia cell lines containing FLT3-ITD mutations with IC50 values of approximately 6 ng/mL.19 Given twice daily by oral gavage, tandutinib increased survival of nude mice with leukemia or lymphoma arising from Ba/F3 cells expressing FLT3-ITD mutations and increased survival of mice with myeloproliferative disease arising from transfection of hematopoietic progenitor cells with such mutations.18,19
Tandutinib has a very limited spectrum of activity outside the type III receptor kinase family. However, in a broad in vitro general pharmacology screen that included various receptor and enzyme assays, tandutinib yielded IC50 values less than 500 ng/mL against the muscarinic nonselective central nervous system acetylcholine receptor (434 ng/mL) and the muscle-type nicotinic acetylcholine receptor (483 ng/mL) (Millennium Pharmaceuticals, data on file). In a competitive human ether-a-go-go related gene (hERG) binding assay, tandutinib had a Ki of 216 ng/mL and an IC50 of 550 ng/mL. In a whole-cell variant of the patch-clamp assay using cells transfected with cloned human cardiac K+ channel hERG, tandutinib had a tail current IC50 of 1742 ng/mL (Millennium Pharmaceuticals, data on file).
Evaluation of tandutinib in rats, dogs, and monkeys showed it to be orally bioavailable, metabolically stable, and most likely eliminated by biliary excretion without biotransformation. Acute administration of high oral doses of tandutinib in dogs produced symptoms suggestive of central nervous system or neuromuscular toxicity, such as lack of coordination and tremors. However, under conditions of chronic oral dosing tandutinib was generally well tolerated in both rats and dogs. The principal toxicologic findings at high chronic doses in both species were (1) mild and reversible hypocellularity of the bone marrow with associated anemia and leukopenia and (2) reversible inflammatory infiltrates in hepatic portal triads, associated with reversible increases in liver function tests (Millennium Pharmaceuticals, data on file).
On the basis of preclinical models showing activity against leukemia cells driven by mutant FLT3 and given tandutinib's relatively favorable in vivo pharmacology and toxicology, a phase 1 clinical trial was initiated to evaluate tandutinib in patients with AML or high-risk myelodysplastic syndrome (MDS).
Patients, materials, and methods
Patients
This study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Ethics Review Committee at each of the 5 participating centers. All patients gave written informed consent to study participation. Patients were eligible if they were older than 18 years and had relapsed or primarily refractory AML, or if they had newly diagnosed AML and were not considered candidates for standard remission induction chemotherapy. In addition, patients with high-risk MDS (refractory anemia with excess of blasts [RAEB] and chronic myelomonocytic leukemia [CMML]) were allowed to participate. Although all patients were assessed for a FLT3-ITD mutation in their leukemic blasts, this mutation was not required for study participation. Patients were required to have an Eastern Cooperative Oncology Group (ECOG) performance status no more than 2 and could not have undergone bone marrow transplantation or peripheral blood stem cell transplantation fewer than 2 months before receiving the first dose of tandutinib. Patients could not have received cytotoxic chemotherapy for 4 weeks prior to the first dose of tandutinib, except hydroxyurea. Hydroxyurea was allowed for up to 10 days after the start of tandutinib dosing. The use of hematopoietic growth factors was not permitted during study therapy, except erythropoietin. Study participation required a serum creatinine level no greater than 176.8 μM (2 mg/dL), a serum bilirubin level no greater than 34.2 μM (2 mg/dL), and hepatic transaminases no more than 3 times the upper limit of normal. Patients were required to have a rate-corrected QT interval (QTc, using Bazett formula) no more than 450 msec.
Treatment protocol
Tandutinib administration. Patients were given tandutinib twice daily by mouth. The medication was provided as 25- or 100-mg capsules, dose strength being expressed as the free base (capsules contained tandutinib as the sulfate salt). Patients were instructed to take no food or drink other than water for 2 hours before and 2 hours after each dose, given preclinical data suggesting that food could decrease oral bioavailability of tandutinib (Millennium Pharmaceuticals, data on file). Patients experiencing neither dose-limiting toxicity (DLT) nor disease progression could remain on therapy for up to 1 year.
Tandutinib dose escalation. The dose selected for initial evaluation was 50 mg twice daily This represents approximately a sixth of the highest nontoxic dose (200 mg/m2 twice daily) in a 28-day toxicology study in dogs (Millennium Pharmaceuticals, data on file). Separate groups of 3 to 6 patients were enrolled to successively higher doses of tandutinib using a modified Fibonacci dose escalation scheme. No dose escalation within individual patients was allowed. New patients could not be enrolled to the next higher dose level of tandutinib until at least 3 patients had been treated at the preceding dose level, had received at least 14 days of treatment with tandutinib, and had not experienced DLT, defined as any grade 3 or 4 nonhematologic toxicity or grade 2 neurologic toxicity. Toxicities were graded according to the National Cancer Institute's Common Toxicity Criteria, version 2.0.
Safety monitoring
Patients were evaluated at the treating centers on days 1, 2, 3, 7, 10, 14, 17, 21, and 28 over the first month of therapy. Serial electrocardiograms (ECGs) were obtained on days 1, 7, 14, and 28, prior to the morning dose of tandutinib. After the first 28 days of therapy, patients were required to return every 14 days for the next 2 months, and monthly thereafter. Each visit was accompanied by a physical examination and evaluation of complete blood count, serum electrolytes, and renal and hepatic function.
Assessment of treatment efficacy
Patients underwent bone marrow biopsy and aspiration during a screening period of up to 2 weeks. Bone marrow biopsy and aspiration were subsequently repeated after completion of the first 28 days of therapy. Patients who continued tandutinib treatment after day 28 underwent repeat bone marrow biopsy and aspiration at least every 60 days and at treatment discontinuation. These results were evaluated in the context of prevailing peripheral blood counts; disease response was assessed according to International Working Group criteria.20
Assessment of FLT3 mutation status
Detection of FLT3-ITD mutations by polymerase chain reaction (PCR). Genomic DNA was obtained from blood or bone marrow samples using a DNAeasy Tissue Kit (Qiagen, Valencia, CA). FLT3 exons 14 and 20 were amplified using the primer pairs 5′-TCTGCAGAACTGCCTATTCCT-3′ (FLT3 sense primer, exon 14), 5′-TTTCCAAAAGCACCTGATCC-3′ (FLT3 antisense primer, exon 14), 5′-GCACTCCAGGATAATACACATCA-3′ (FLT3 sense primer, exon 20), 5′-AACGACACAACACAAAATAGCCG-3′ (FLT3 antisense primer, exon 20). PCR amplification of genomic DNA was performed using 500 ng DNA.
Denaturing wave high-performance liquid chromatography (D-HPLC). Aliquots (5-20 μL) of each PCR reaction were assessed for FLT3 mutations using a Transgenomic WAVE HPLC system (Transgenomic, Omaha, NE). Samples were run at 50°C to distinguish fragments of different lengths in exon 14 and at 56.9°C (exon 14) and 59.1°C (exon 20) to detect point mutations. Amplimers with abnormal D-HPLC profiles were bidirectionally sequenced on an ABI 310 Sequencer using the BigDye Terminator Kit (Applied Biosystems, Foster City, CA). Mutation gene dosage was determined by integration of the mutant-specific HPLC peak area and comparison of the calculated percentage mutant allele versus blast percentage. Samples for which the ratio of calculated percentage mutant allele to blast percentage greater than 0.75 were judged to be homozygous or hemizygous for the FLT3-ITD mutation.
Pharmacokinetics
Peripheral blood samples for determination of tandutinib plasma concentrations were obtained at 0.25, 0.5, 1, 2, 4, 6, and 8 hours following the first dose of tandutinib. Additional samples were obtained before the morning dose on days 3, 7, 10, 14, 17, and 21. On day 28, samples were obtained before the morning dose and at 0.25, 0.5, 1, 2, 4, 6, and 8 hours after that dose. Patients did not resume tandutinib dosing until day 31; additional blood samples were collected on days 29, 30, and 31. This was the only planned dosing interruption during the study. Tandutinib plasma concentrations were determined using a validated liquid chromatography-mass spectrometry method.
The pharmacokinetics of tandutinib were evaluated with nonlinear mixed effects modeling, using NONMEM (version V level 1.1)21 and Wings for NONMEM,22 running under Compaq Visual Fortran (version 6.6c). The first-order conditional estimation (FOCE) method with interaction was applied to all analyses. Model acceptance was based on successful minimization, significant reduction in objective function values, diagnostic plots, and a posterior predictive check procedure.
Pharmacodynamics
Peripheral blood samples were collected to assess the effect of tandutinib on the activation (phosphorylation) of FLT3 in patients' leukemic blasts. Samples were obtained on day 1 prior to, and 2 and 8 hours after, the first dose of tandutinib. Additional samples were obtained prior to dosing on days 3, 10, and 28. Blood was collected in 4-mL CPT Vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ). After centrifugation supernatants containing mononuclear cells were transferred to Eppendorf tubes, and cells were pelleted. Serum was removed and cell pellets were immediately frozen on dry ice.
Immunoprecipitation. Frozen cell pellets were lysed in 500 μL freshly made lysis buffer, and total FLT3 was immunoprecipitated from cell lysate supernatants by addition of 3 μg anti-FLT3 polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA; catalog no. SC-480) followed by protein-A agarose beads (Pierce, Rockford, IL; catalog no. 20333). Beads were washed twice with 1 mL cold lysis buffer before the addition of 40 μL/tube of sodium dodecyl sulfate (SDS) protein loading buffer. Tubes were boiled for 5 minutes, and supernatants were loaded onto 12% to 20% Tris-glycine SDS-polyacrylamide gel for electrophoresis. Proteins were transferred to nitrocellulose membranes for 1.5 hours at 125 V.
Phosphorylated FLT3 (pFLT3) detection. Blots were incubated in blocking buffer (5% nonfat dry milk in 1X Tris-buffered saline [TBS] containing 0.5% Tween 20) for 1 hour at ambient temperature, washed 3 times in washing buffer (1X TBS containing 0.5% Tween 20), and incubated overnight with mouse anti-pFLT3 monoclonal antibodies (diluted 1:1000 in blocking buffer). After washing, blots were incubated with horseradish peroxidase (HRP)–conjugated goat anti–mouse IgG (Biosource International, Camarillo, CA; catalog no. AMI4404) for 1 hour at ambient temperature. Blots were washed again prior to incubation with enhanced chemiluminescence (ECL) substrate (Amersham Bioscience, Piscataway, NJ; catalog no. RPN2106) for 1 minute at ambient temperature and were rapidly exposed to X-ray films (Hyperfilm; Amersham; catalog no. RPN3114K).
Total FLT3 detection. Blots were stripped of antibodies by incubation in Western Stripping buffer (Pierce; catalog no. 21059) for 30 minutes at room temperature. After washing, blots were incubated for 30 minutes in blocking buffer. Total FLT3 was detected by anti-FLT3 polyclonal antibodies (Santa Cruz Biotechnology; catalog no. SC-480) for 1 hour at ambient temperature, followed by sheep anti–rabbit IgG-HRP (Chemicon International, Temecula, CA; catalog no. AP304P). Blots were washed, incubated with ECL substrate, and exposed to X-ray film, as described in “Phosphorylated FLT3 (pFLT3) detection.”
The qualitative assessment of FLT3 phosphorylation was done visually. Because the amount of protein loaded onto the gels was not standardized for every time point, the intensity of each pFLT3 band was normalized to that of the corresponding total FLT3 band at each time point. Inhibition of FLT3 phosphorylation was then assessed with reference to the intensity of the bands in the baseline sample. Given the semiquantitative nature of the assay and the qualitative analysis, results were categorized as “no inhibition,” “inhibition,” or “not assessable” (eg, no detectable band in the predose sample or samples without detectable total FLT3).
Results
Patient characteristics
Between May 2002 and June 2003, 40 patients were enrolled (Table 1). Only 16 (40%) patients had ever achieved a complete response with any previous treatment, 15 (38%) patients had AML that arose from preexisting myelodysplasia, and 6 (17%) of 35 patients had unfavorable cytogenetics. Eight patients were found to have FLT3-ITD mutations (Table 2). The blast cells from 4 of these 8 patients were determined to contain only the mutant allele (homozygous or hemizygous). One additional patient had a point mutation (D835Y) in the second tyrosine kinase domain of the receptor.
Tandutinib dose escalation
Table 3 summarizes the tandutinib dose levels evaluated. The initial dose level (50 mg twice daily) required expansion to 6 patients because 1 of the first 3 patients died on day 14. This patient had a sudden cardiorespiratory arrest and, although the patient had underlying chronic obstructive pulmonary disease, it was considered prudent to further evaluate the safety of this initial dose before continuing dose escalation. The 150-mg twice daily dose level required expansion to 6 patients because 1 of the first 3 patients developed a small bowel obstruction after 15 days of treatment, for which there was no alternative clinical explanation; this resolved with nasogastric suctioning. The 300-mg twice daily dose level was expanded to 6 patients after 1 of the first 3 patients developed overwhelming sepsis on day 13 of treatment as well as bowel obstruction in the context of a previous history of typhilitis and partial colonic resection. The 400-mg twice daily dose level enrolled 4 patients because the first patient enrolled developed atrial fibrillation and hypoxemia after 1 dose of tandutinib. No further tandutinib therapy was given to this patient, and he was replaced.
At the 525-mg twice daily dose level, 1 of the first 3 patients enrolled terminated dosing on day 10 because of grade 3 generalized muscular weakness and fatigue. The patient had a preexisting benign intention tremor that worsened, and she also developed hyperreflexia with clonus. There was no change in mental status, speech, or affect, and there were no focal abnormalities on neurologic examination. At the time of these observations the tandutinib plasma concentration was 1060 ng/mL. The patient's weakness improved to grade 1 to 2 within 72 hours of stopping tandutinib therapy. Complete resolution of all abnormal findings could not be documented because the patient was subsequently transferred to hospice care with progressive leukemia. In light of this DLT, 3 additional patients were enrolled to the 525-mg twicedaily dose level. None of these additional patients experienced DLT or weakness or fatigue related to tandutinib.
Three patients were enrolled to the 700-mg twice-daily dose level. Two of these patients terminated tandutinib therapy on days 9 and 16, respectively, because of grade 3 and 4 generalized muscular weakness. The corresponding trough plasma concentrations of tandutinib in these 2 patients were 1390 and 2220 ng/mL. No focal neurologic deficits were demonstrable in either patient, and neither patient had any change in mental status, speech, or affect. In contrast to the patient experiencing dose-limiting weakness at 525 mg twice daily, neither of these patients exhibited hyperreflexia or tremor. The patient with grade 3 weakness fully recovered within 24 hours of stopping tandutinib, and the patient with grade 4 weakness recovered after 3 days.
In the 3 cases of tandutinib-related muscular weakness, the tandutinib plasma concentration determined 12 hours or more after the last dose exceeded 1000 ng/mL. Only 1 other patient, treated at 400 mg twice daily, had a similarly high plasma concentration. On day 14 of treatment this patient's predose tandutinib plasma concentration was 1010 ng/mL. A review of this patient's dosing history indicated that the day 14 sample was taken before that morning's dose of tandutinib. However, unlike the patients who developed dose-limiting muscular weakness, the preceding trough tandutinib plasma concentrations in this patient were significantly lower (577 ng/mL on day 10), and, as tandutinib therapy continued, plasma concentrations remained well below 1000 ng/mL (432 ng/mL on day 17).
Safety
Toxicities other than muscular weakness were observed with tandutinib treatment (Table 4). Tandutinib treatment was associated with nausea and vomiting and less often with diarrhea. These symptoms were usually manageable with standard antiemetics (5-HT3 antagonists, lorazepam) and antidiarrheal medications (loperamide). However, tandutinib tended to exacerbate preexisting nausea, vomiting, or diarrhea and produced dose-limiting (grade 3) diarrhea in 1 patient treated at 700 mg twice daily. Several patients developed lower-extremity or periorbital edema while taking tandutinib. This was manageable with symptomatic measures and diuretic therapy.
Although mild myelosuppression was observed in preclinical toxicology studies with the chronic administration of tandutinib at high doses, no evidence of hematologic toxicity was observed in this study. Decreases in peripheral blood cell counts were always accompanied by increases in bone marrow blast counts. Four patients without FLT3-ITD mutations maintained stable peripheral blood counts and bone marrow blast counts for relatively long periods of time, ranging from 154 to 190 days, at tandutinib doses ranging from 100 to 525 mg twice daily.
Preclinical evaluation of tandutinib suggested that it may have the potential for prolongation of the QT interval. However, it was not possible in this complex, often acutely ill patient population to rigorously assess the effect of tandutinib on the QT interval. Nevertheless, a regression analysis (Figure 1) was performed to explore the relation between tandutinib dose and change in QTc from baseline, measured after 28 days of tandutinib administration. The day 28 time point was chosen because at earlier time points tandutinib plasma concentrations were generally lower. Linear regression analysis showed the slope of the line to be 0.064, suggesting that for each 100-mg increase in the dose of tandutinib there was a 6.4-msec increase in QTc compared with baseline. However, although the slope of the line is positive, it is not statistically different from zero (P = .240, t test). Further analysis revealed that 1 patient treated at 525 mg twice daily, who had a 270-msec increase in QTc on day 28, is responsible for the positive slope of the linear regression line. Without the inclusion of this patient the slope is slightly negative (–0.007), although not statistically different from zero (P = .812, t test). Interestingly, this patient's profound QT interval prolongation on day 28 escaped initial clinical detection, and he continued therapy with tandutinib for a total of 162 days. His QT interval returned to within normal limits as dosing continued. In addition, this patient's tandutinib plasma concentrations were much below the population average throughout his course of treatment: on day 28 his predose tandutinib plasma concentration was only 54 ng/mL, with a maximum of 156 ng/mL measured 1 hour after dosing.
Change in day-28 rate-corrected QT interval (QTc) from baseline versus tandutinib dose.
Change in day-28 rate-corrected QT interval (QTc) from baseline versus tandutinib dose.
Pharmacokinetics
Figure 2A-B shows an example of a representative tandutinib plasma concentration versus time profile. Figure 2A shows the profile over the course of treatment and Figure 2B shows the profile on day 28. The dose was 525 mg twice daily. By study design, sampling was adequate to describe the concentration versus time profile following the first dose on day 1 and the dose on day 28 (day 27 on the plot; first dose is depicted on day 0). Intervening sampling was for trough concentrations only. The dip in trough concentration on day 13 is the result of a missed dose.
The tandutinib plasma concentration versus time curve following the day 28 dose shows 2 phases of plasma concentration versus time decay. This observation was subsequently corroborated by modeling, whereby a 2-compartment open linear model with first-order absorption best described the pharmacokinetic data. Estimates of absolute pharmacokinetic parameter values could not be made because intravenous tandutinib administration data were not available. Therefore, the pharmacokinetic parameters were calculated relative to absolute bioavailability (F). The parameters determined were relative total body clearance (CL/F), intercompartmental clearance (Q2/F), apparent central volume of distribution (Vc/F), apparent peripheral volume of distribution (Vp/F), mean residence time (MRT), absorption half-life (t1/2 abs), and lag time (Tlag) (Table 5).
Tandutinib plasma concentration versus time profile for a patient receiving 525 mg twice daily for 28 days. (A) Plasma concentration versus time profile during days 0 to 30. (B) Plasma concentration versus time profile following completion of dosing. Dip in trough concentration on day 13 because of missed dose. Day 27 on the plot corresponds to day 28 of dosing, because for the purposes of the plot the beginning of day 1 is viewed as “time-zero” in units of days. Concentrations on study day 1 and following the day 28 dose result from intense sampling, enabling viewing of the full concentration versus time profile. Other concentrations represent troughs only.
Tandutinib plasma concentration versus time profile for a patient receiving 525 mg twice daily for 28 days. (A) Plasma concentration versus time profile during days 0 to 30. (B) Plasma concentration versus time profile following completion of dosing. Dip in trough concentration on day 13 because of missed dose. Day 27 on the plot corresponds to day 28 of dosing, because for the purposes of the plot the beginning of day 1 is viewed as “time-zero” in units of days. Concentrations on study day 1 and following the day 28 dose result from intense sampling, enabling viewing of the full concentration versus time profile. Other concentrations represent troughs only.
The large value for CL/F (148 L/h/70 kg) implies that the extent of tandutinib systemic uptake is incomplete, perhaps because of incomplete absorption; antitransport; or first-pass, extrahepatic elimination, or both. If tandutinib is distributed equally in plasma and red cells and liver blood flow is 90 L/h, then an estimate of the extent of uptake into the systemic circulation would be 21%. The 2-compartment model population parameters predict that, on average, 90% and 95% of steady-state plasma concentrations are achieved after 8.6 and 11.4 days of treatment, respectively.
Pharmacodynamics
To examine the effect of tandutinib on the phosphorylation (activation) of either wild-type or ITD-mutated FLT3, peripheral blood blasts were isolated from patients before and after tandutinib administration. The phosphorylation state of FLT3 was examined using Western blot analysis, probing with antibodies specific for total and phosphorylated FLT3. Four patients were evaluable; 1 had wild-type FLT3, and the other 3 carried FLT3-ITD mutations. The remaining 36 patients had insufficient numbers of circulating blasts to produce FLT3 levels above the detection limit of the assay. Because of the limited dynamic range and low sensitivity of the assay, a quantitative analysis of the relationship between tandutinib plasma concentration and FLT3 phosphorylation could not be performed.
The patient with wild-type FLT3 received tandutinib at 150 mg twice daily. Total FLT3 levels in this patient were comparable to levels in patients with ITD mutations. However, the level of pFLT3 prior to the first dose of tandutinib was very low and did not change through day 3 (last data point available), making assessment of inhibition difficult. The tandutinib plasma concentrations for this patient were 23.3, 6.4, and 13.3 ng/mL at 2 and 8 hours, day 1, and day 3, respectively, all well below the concentration predicted necessary for inhibition of receptor phosphorylation.
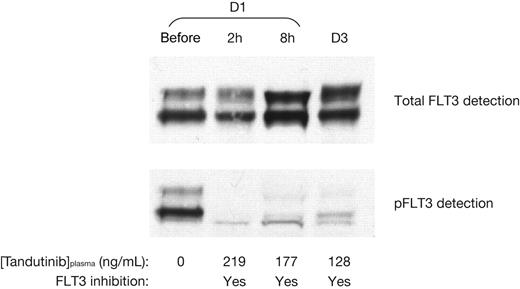
The patients with FLT3-ITD mutations received tandutinib at 300 mg (1 patient) and 525 mg (2 patients) twice daily. Figure 3 depicts the total FLT3 and pFLT3 status for 1 of the patients treated at 525 mg twice daily, prior to tandutinib administration, 2 and 8 hours after the first dose, and prior to the morning dose of tandutinib on day 3. Prior to tandutinib administration, both total FLT3 and pFLT3 can be readily detected. Subsequently, with associated tandutinib plasma concentrations of 219, 177, and 128 ng/mL, total FLT3 is unchanged to increased, whereas pFLT3 is clearly reduced. Reduction in pFLT3 compared with prior to dosing was also observed at different time points in the presence of tandutinib in the other patients with FLT3-ITD mutations, showing that tandutinib has an inhibitory effect on FLT3 phosphorylation (Table 6).
Clinical activity
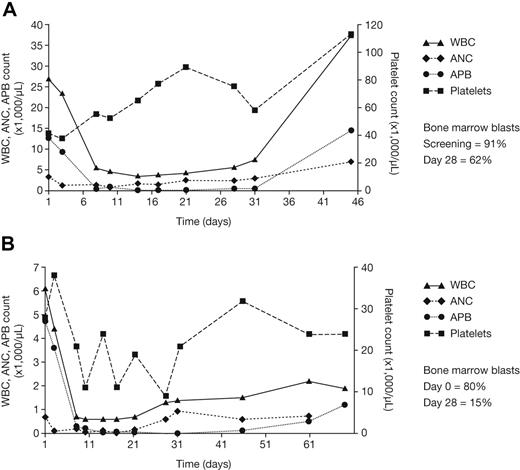
No complete or partial remissions were observed in this study. However, 2 of 8 patients with FLT3-ITD mutations, treated at 525 mg and 700 mg twice daily, respectively, exhibited evidence of an antileukemic effect (Figure 4). No significant antileukemic effects other than stable disease were noted in patients with wild-type FLT3. One patient with a FLT3-ITD mutation treated at 525 mg twice daily experienced a greater than 99% decrease in absolute peripheral blast count and a decrease in bone marrow blast percentage from 91% to 62% over the first 28 days of therapy. Although there was no improvement in absolute neutrophil count (ANC), there was a modest increase in platelet count from 40 × 109 to 70 × 109/L (40 to 75 ×103) over this period. By day 44 this patient showed evidence of disease progression, and tandutinib therapy was discontinued. Another patient with a FLT3-ITD mutation treated at 700 mg twice daily likewise experienced a greater than 99% decrease in absolute peripheral blast count and a decrease in bone marrow blast percentage from 80% to 15% by day 28. No significant improvement was observed in ANC or platelet count. After 2 months of therapy this patient also experienced disease progression.
Among the remaining 6 patients with FLT3-ITD mutations, a number of factors potentially precluded or confounded the observation of an antileukemic effect. One patient was treated at 150 mg twice daily and did not achieve tandutinib plasma concentrations expected to consistently inhibit receptor activation. A patient treated at 300 mg twice daily developed overwhelming sepsis on day 13 of treatment, prompting cessation of tandutinib dosing. Although this patient's absolute peripheral blast count was decreasing when tandutinib dosing was stopped, the patient was also receiving therapy with hydroxyurea. As described earlier, another patient with a FLT3-ITD mutation was given a single 400-mg dose of tandutinib and within hours developed atrial fibrillation and hypoxemia considered to be unrelated to study therapy; the decision was made to not continue protocol therapy. Two patients, 1 treated at 525 mg twice daily and the other treated at 700 mg twice daily, stopped therapy on days 10 and 9, respectively, because of tandutinib-induced weakness. Neither of these patients resumed treatment with tandutinib, thereby precluding response evaluation. An additional patient treated at 525 mg twice daily withdrew from the study on day 32 and, although evaluable for response, there was no evidence of an antileukemic effect.
Relation between inhibition of FLT3 phosphorylation in peripheral blasts and plasma concentration of tandutinib in a single patient receiving 525 mg twice daily. D1 indicates day 1; D3, day 3.
Relation between inhibition of FLT3 phosphorylation in peripheral blasts and plasma concentration of tandutinib in a single patient receiving 525 mg twice daily. D1 indicates day 1; D3, day 3.
One patient had a D835Y point mutation in the activation loop of FLT3. After 31 days of tandutinib therapy at 400 mg twice daily, this patient's leukemia had progressed.
Discussion
Although preclinical toxicology data suggested that myelosuppression and hepatic inflammation would be the main limitations of tandutinib therapy, the dose-limiting toxicity of tandutinib proved to be generalized muscular weakness, fatigue, or both. The patient who developed weakness at the 525-mg twice-daily dose level had generalized hyperreflexia with clonus, which suggested a centrally mediated effect. However, no other signs or symptoms of central nervous system toxicity were manifest in this patient, and none were manifest in either of the 2 patients treated at 700 mg twice daily who developed generalized muscular weakness. The current hypothesis is that this toxicity may result from an effect of tandutinib at the neuromuscular junction, as suggested by preclinical data showing that tandutinib has the capacity to bind to a muscle-type nicotinic receptor. This toxicity does not appear to be related to tandutinib's inhibition of FLT3, KIT, or PDGFR, given that muscular weakness has not been reported with other FLT3 antagonists in clinical development.23-25
Despite the slow elimination of tandutinib, tandutinib-induced muscular weakness proved to be rapidly reversible. The time course required for resolution of this toxicity is likely explained by tandutinib's biphasic pharmacokinetic profile: following oral dosing and achievement of maximum plasma concentration there is an initially rapid decline in tandutinib plasma concentration, reflecting drug distribution, followed by a much slower phase of drug elimination (Figure 2B). All 3 patients presenting with muscular weakness were found to have trough tandutinib plasma concentrations greater than 1000 ng/mL. They undoubtedly had higher plasma concentrations immediately after tandutinib dosing. Only one other patient in this trial exhibited a trough concentration greater than 1000 ng/mL, but this concentration (1010 ng/mL) was achieved only transiently. These observations have led to the provisional hypothesis that the general muscular weakness associated with tandutinib is related to its plasma concentration, and that trough concentrations of 1000 ng/mL or greater should be avoided. However, the limited data regarding the ability of tandutinib to inhibit the activation (phosphorylation) of either wild-type or ITD-mutated FLT3 are consistent with preclinical data, suggesting that the in vivo IC90 is 150 ng/mL or greater.18,19 Therefore, assuming that the goal of therapy is to continuously maintain plasma concentrations of IC90 or greater, the therapeutic index of tandutinib is 1000/150 = 6.7.
Single-patient hematologic data for 2 patients treated with tandutinib. The patients received (A) tandutinib 525 mg twice daily or (B) tandutinib 700 mg twice daily. WBCs indicate, white blood cells; ANC, absolute neutrophil count; APB, absolute peripheral blasts.
Single-patient hematologic data for 2 patients treated with tandutinib. The patients received (A) tandutinib 525 mg twice daily or (B) tandutinib 700 mg twice daily. WBCs indicate, white blood cells; ANC, absolute neutrophil count; APB, absolute peripheral blasts.
Tandutinib therapy was associated with other toxicities that, although not dose limiting, are clinically important. In the majority of patients, tandutinib-related nausea, vomiting, and diarrhea were grade 1 in severity and could be managed successfully with standard supportive therapies such as 5-HT3 antagonists and loperamide. However, tandutinib tended to exacerbate preexisting nausea, vomiting, or diarrhea and resulted in one instance of dose-limiting diarrhea. The periorbital and peripheral edema associated with tandutinib therapy were mild and manageable and are mainly of interest because similar edema is observed with imatinib mesylate.2-5 The edema associated with imatinib mesylate has been attributed to PDGFR inhibition, and tandutinib is a potent inhibitor of PDGFR.19 Although no relation could be found between tandutinib dose and change from baseline in length of the QTc interval, this analysis should not be viewed as conclusive. Definitive conclusions about the possible effect of tandutinib on the QT interval will require dedicated studies in more stable patients or in healthy subjects.
This phase 1 trial was limited in its ability to assess the antileukemic activity of tandutinib. The majority of patients were treated at doses not expected to be effective, and only 8 patients in the study had AML with FLT3-ITD mutations. Only 1 patient with an activating point mutation in FLT3 was treated in this study, and in this patient there was no evidence of an antileukemic effect. Although no conclusions can be drawn from this experience, tandutinib is known to have lower potency against activating point mutations in FLT3 than against ITD mutations.26 Even among the patients with FLT3-ITD mutations who were treated at potentially effective doses, response evaluation was often not possible because of rapid disease progression, sudden disease-related clinical deterioration, or tandutinib-related toxicity. Nevertheless, evidence of antileukemic activity was observed in 2 patients treated at 525 and 700 mg twice daily, respectively. This activity did not fulfill the traditional, protocol-specified definition of a partial or complete remission, which perhaps is not surprising given the patient population and the complexity of this disease. However, combined with the evidence that tandutinib inhibits the activation (phosphorylation) of FLT3 in patients' leukemic blasts, the observed activity provides hope that phase 2 testing of tandutinib 525 mg twice daily in patients with AML and FLT3-ITD mutations may confirm the therapeutic activity of this agent.27
Authorship
D.J.D. designed and performed research, analyzed data, and assisted in the final preparation of the manuscript; R.M.S. and M.A.C. designed and performed research; M.L.H. and R.B.K. designed and performed research and analyzed data; S.D.N. designed and performed research and edited the paper; R.L.P. and P.T.C. performed research; M.R.C. was medical monitor for the trial, analyzed the clinical data, and wrote initial drafts of the manuscript; J.-M.L. performed research, analyzed data, and wrote a section of the paper; M.D.K. performed the pharmacokinetic analysis, contributed to the pharmacokinetic results, and reviewed the paper; S.S. was the biostatistician for trial, analyzed and reviewed data, and reviewed the paper; N.H. performed the pharmacokinetic analysis and contributed details of pharmacokinetic methods and results for the manuscript; B.J.D. performed research and analyzed data; M.C.H. designed and performed research, contributed vital new reagents or analytical tools, analyzed data, and wrote a section of the paper.
D.J.D., R.M.S., M.L.H., S.D.N., R.L.P., R.B.K., M.A.C., P.T.C., and B.J.D. have no commercial interests to disclose. M.R.C., J.-M.L., M.D.K., and S.S. are employed by Millennium Pharmaceuticals Inc, whose product was studied in the present work. N.H. and M.C.H. are consultants to Millennium Pharmaceuticals Inc, whose product was studied in the present work. M.C.H. is also a consultant to Novartis Pharmaceuticals.
Prepublished online as Blood First Edition Paper, August 14, 2006; DOI 10.1182/blood-2006-02-005702.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Laura McGreevey and Tina Harrell for assisting with the FLT3 mutational analyses, Ilene Galinksy (D.J.D. and R.M.S.), and Jeffrey Gardner for technical assistance (M.L.H.). We also thank Steve Hill (medical writer) and Rachel Higgins (editor with Gardiner-Caldwell London) for their support in drafting the manuscript.
This work was supported in part by research funding from Millennium Pharmaceuticals (grant POI CA66996-06A1), by a Veterans Administration (VA) Merit Review Grant (M.C.H.), aLeukemia and Lymphoma Society Specialized Center of Research (LLS SCOR) grant (S.D.N.), and a Doris Duke Charitable Foundation Distinguished Clinical Scientist Development Award (B.J.D.).