Abstract
The essential event in platelet adhesion to the injured blood vessel wall is the binding to subendothelial collagen of plasma von Willebrand factor (VWF), a protein that interacts transiently with platelet glycoprotein Ibα (GPIbα), slowing circulating platelets to facilitate firm adhesion through collagen receptors, including integrin α2β1 and GpVI. To locate the site in collagen that binds VWF, we synthesized 57 overlapping triple-helical peptides comprising the whole triple-helical domain of collagen III. Peptide no. 23 alone bound VWF, with similar affinity to that of native collagen III. Immobilized peptide no. 23 supported platelet adhesion under static and flow conditions, processes blocked by an antibody that prevents collagen from binding the VWF A3 domain. Truncated and alanine-substituted peptides derived from no. 23 either strongly interacted with both VWF and platelets or lacked both VWF and platelet binding. Thus, we identified the sequence RGQOGVMGF (O is hydroxyproline) as the minimal VWF-binding sequence in collagen III.
Introduction
The interaction of collagen with von Willebrand factor (VWF) requires unique structural properties in both proteins. Optimal hemostatic function requires multimerization of up to 50 VWF monomers in circulating plasma; higher-order multimers bind collagen more tightly than smaller assemblies of VWF.1 Several collagens occur in the vessel wall, of which collagens I and III are considered most important in supporting platelet adhesion to the damaged vasculature.2 We have identified the residues in the VWF A3 domain that bind collagen III, using site-directed mutagenesis guided by the crystal structure of the VWF A3 domain in complex with a monoclonal antibody (RU5) that inhibits its interaction with collagen.3,4 Nishida et al mapped the collagen-binding mode of the A3 domain by nuclear magnetic resonance and confirmed results by site-directed mutagenesis.5 However, the VWF-binding site(s) in collagen is unknown, although progress in understanding how collagen interacts with integrin α2β1 and GpVI has been made using short synthetic triple-helical peptide analogues of collagen,6,7 including the Collagen III Toolkit.8 We used the same approach to identify the high-affinity VWF-binding site in human collagen III, information that may help to develop the collagen-VWF interaction as an antithrombotic target.9,10
Materials and methods
Peptide synthesis
The synthesis and characterization of the 57 overlapping triple-helical peptides of the Collagen III Toolkit (Table S1, available at the Blood website; see the Supplemental Materials link at the top of the online article) is detailed elsewhere.8 The same approach was used to synthesize and verify derivative peptides (Table S2). The sequence of peptide no. 23 is GPC-(GPP)5-GPOGPSGPRGQOGVMGFOGPKGNDGAO-(GPP)5-GPC-NH2, and of the minimal VWF-binding derivative peptide, GPC-(GPP)5-GPRGQOGVMGFO-(GPP)5-GPC-NH2.
Static platelet-binding assay
Blood was obtained from the antecubital vein of informed volunteers, in accordance with the Helsinki protocol, into 0.105-M citrate Vacutainers (Becton Dickinson, Oxford, United Kingdom). Platelet-rich plasma was prepared after 2 spins for 1 minute at 1200g. Then, 10% (vol/vol) of ACD buffer (39 mM citric acid, 75 mM trisodium citrate, 135 mM d-glucose, pH 4.5) and prostaglandin E1 (280-nM final concentration) were added, and the platelets were pelleted for 12 minutes at 700g, then resuspended in 6 mL buffer (5.5 mM d-glucose, 128 mM NaCl, 4.26 mM Na2HPO4, 7.46 mM NaH2PO4, 4.77 mM trisodium citrate, 2.35 mM citric acid, 0.35% bovine serum albumin [BSA], pH 6.5). Prostaglandin E1 was added similarly, and the platelets were spun for 6 minutes at 700g. Platelets were resuspended to 1.25 × 108 platelets/mL in adhesion buffer (0.05 M Tris-HCl, 0.14 M NaCl, 0.1% BSA, pH 7.4), and the adhesion of platelets from 100-μL portions was determined colorimetrically, as described.11 Peptides (1 μg/well) were coated onto Immulon-2-HB plates (Nunc, Rochester, NY), conditions supporting maximal platelet binding to peptide no. 23. The αIIbβ3 antagonist, GR144053F (2 μM; Calbiochem, Nottingham, United Kingdom), or antibody RU5 (2 μg/mL) was preincubated for 15 minutes with platelets, where indicated. Approval for these studies was obtained from the University of Cambridge Human Biology Research Ethics Committee.
Solid-phase binding assays
To measure binding of VWF12 (purified from Haemate P; Aventis-Behring, Hattersheim am Main, Germany), 96-well plates were coated with peptides (1 μg/well) or collagen III (10 μg/well; Sigma, St Louis, MO) and incubated with VWF (1 μg/mL), which was detected with a polyclonal antibody.12
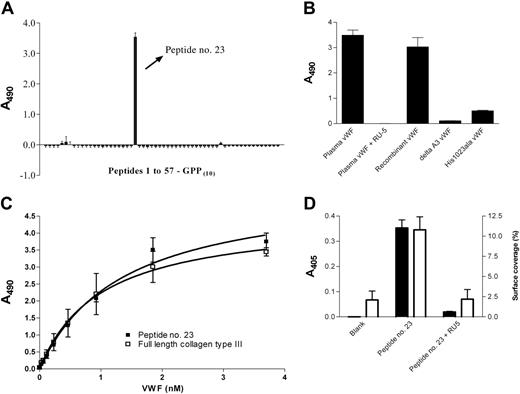
Identification of a single peptide containing 27 amino acids of collagen III sequence that specifically binds VWF. (A) The peptides (10 μg/mL) of the Collagen III Toolkit (Table S1) were each immobilized on a 96-well plate, and the adhesion of plasma-derived VWF (1 μg/mL) was determined. (B) Immobilized peptide no. 23 was incubated with plasma-derived VWF (1 μg/mL) in presence or absence of monoclonal antibody RU5 (1 μg/mL), or with recombinant wild-type, delta-A3, or His1023Ala VWF (all at 1 μg/mL). In panels A-B, bound VWF was detected as described in “Solid-phase binding assays,” and the mean ± SD is shown from a representative of 3 independent experiments, each performed in duplicate. (C) Immobilized peptide no. 23 or immobilized collagen III (100 μg/mL) was incubated with increasing concentrations of VWF, detected as in panel A. (D) Peptide no. 23 or vehicle was coated in a 96-well plate (10 μg/mL) or sprayed onto a coverslip (0.5 μg/cm2) and incubated with washed platelets or perfused with whole blood for 5 minutes at a shear rate of 300 s–1 in presence or absence of RU5 (1 μg/mL). Uncoated wells or coverslips served as controls. The adhesion of platelets bound in the static assay (▪) and the surface coverage of platelets (□) were each determined as described in “Static platelet-binding assay” and “Solid-phase binding assays.” Shown is the mean ± SD of 3 independent experiments each performed in triplicate.
Identification of a single peptide containing 27 amino acids of collagen III sequence that specifically binds VWF. (A) The peptides (10 μg/mL) of the Collagen III Toolkit (Table S1) were each immobilized on a 96-well plate, and the adhesion of plasma-derived VWF (1 μg/mL) was determined. (B) Immobilized peptide no. 23 was incubated with plasma-derived VWF (1 μg/mL) in presence or absence of monoclonal antibody RU5 (1 μg/mL), or with recombinant wild-type, delta-A3, or His1023Ala VWF (all at 1 μg/mL). In panels A-B, bound VWF was detected as described in “Solid-phase binding assays,” and the mean ± SD is shown from a representative of 3 independent experiments, each performed in duplicate. (C) Immobilized peptide no. 23 or immobilized collagen III (100 μg/mL) was incubated with increasing concentrations of VWF, detected as in panel A. (D) Peptide no. 23 or vehicle was coated in a 96-well plate (10 μg/mL) or sprayed onto a coverslip (0.5 μg/cm2) and incubated with washed platelets or perfused with whole blood for 5 minutes at a shear rate of 300 s–1 in presence or absence of RU5 (1 μg/mL). Uncoated wells or coverslips served as controls. The adhesion of platelets bound in the static assay (▪) and the surface coverage of platelets (□) were each determined as described in “Static platelet-binding assay” and “Solid-phase binding assays.” Shown is the mean ± SD of 3 independent experiments each performed in triplicate.
Platelet binding under flow conditions
Peptides (0.5 μg/cm2) or collagen III (6.5 μg/cm2) was spray-coated onto Thermanox coverslips (Nunc), then blocked with 1% human albumin in PBS and perfused at 22°C with citrated whole blood for 5 minutes at a shear rate of 300 s–1 using a single-pass perfusion chamber,13 and platelet adhesion was evaluated.14 The institutional review board of the University Medical Centre, University of Utrecht, approved these studies.
Surface plasmon resonance
A Biacore 2000 system (Biacore AB, Uppsala, Sweden) was used for surface plasmon resonance analysis (SPR). Peptides were immobilized on a CM5 sensor chip via the cysteine-free thiols in the peptide N- and C-termini, using the manufacturer's procedures. Binding to a channel coated with control peptide, GPC-(GPP)10-GPC was used to correct the binding of VWF or A3 domain to collagen- or other peptide-coated channels, and VWF concentration used to calculate affinities was based on that of the monomer, measured by enzyme-linked immunosorbent assay.
Results and discussion
Mapping the VWF-binding site within collagen III
A single Collagen III Toolkit peptide, no. 23, bound plasma-derived human VWF (Figure 1A), a process that resembled VWF binding to collagen: (1) the interaction was abolished by a monoclonal antibody, RU5,3 directed against the collagen-binding site of VWF A3 domain (Figure 1B); (2) dysfunctional recombinant variants of VWF, delta A3 VWF15 (which lacks the A3 domain) and His1023Ala VWF3 (in which a crucial A3 amino acid is substituted), showed severely reduced binding to peptide no. 23 (Figure 1B); (3) VWF had similar affinity for peptide no. 23 as for full-length collagen III measured in a solid-phase assay (Figure 1C); and (4) peptide no. 23 bound washed human platelets in static assays, most likely reflecting the presence on the platelet surface of residual plasma-derived VWF or VWF inevitably secreted during platelet preparation, and supported platelet deposition from flowing whole blood at low shear rate (300 s–1; Figure 1D). The high density (20-fold that of native collagen) of VWF-binding sites on the peptide-coated surface may be responsible for the static binding activity, and the complete blockade of these events by RU5 shows the fundamental role of VWF. Under static conditions, antagonism of either integrin αIIbβ3 or GpVI slightly impaired platelet binding to peptide no. 23, consistent with minor involvement of other receptors but with GPIb being the main VWF receptor (data not shown).
Identification of the minimal collagen sequence required for VWF binding
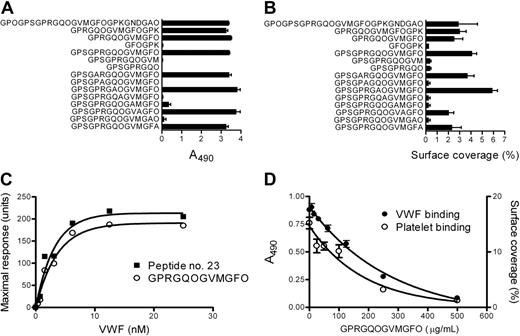
A set of truncated triple-helical derivatives of peptide no. 23 was synthesized, including an alanine-scanned set, peptides 7 to 14 (Table S2). Peptides either bound VWF (Figure 2A) and platelets (Figure 2B) strongly or completely lacked affinity for both VWF and platelets. Thus we identified RGQOGVMGF as the minimum sequence within collagen III required to bind VWF. Residues R, O, V, and F appear crucial for VWF binding, but not Q and M. The triple-helical peptide GPC-(GPP)5-GPRGQOGVMGFO-(GPP)5-GPC-NH2, its parent peptide no. 23, and full-length collagen bound VWF in solid-phase assays with similar affinity (data not shown). SPR allowed more detailed kinetic analysis. A recombinant VWF A3 domain16 bound the immobilized peptides with modest affinity (Kd, 1.8 μM for peptide no. 23; Kd, 2.5 μM for GPC-(GPP)5-GPRGQOGVMGFO-(GPP)5-GPC-NH2; data not shown), whereas full-length, plasma-derived VWF displayed much higher affinity, attributable to its multimeric nature (Kd, 2.1 nM for peptide no. 23; Kd, 2.5 nM for GPC-(GPP)5-GPRGQOGVMGFO-(GPP)5-GPC-NH2; Figure 2C). These affinity constants are consistent with those observed for A3 and VWF binding to full-length collagen12,17 and imply a single VWF-binding site within collagen III.
Identification of the minimal collagen sequence required for VWF binding. (A) The truncated and alanine-modified peptides derived from peptide no. 23 (Table S2) were immobilized on a 96-well plate and incubated with plasma-derived VWF (1 μg/mL), and bound VWF was detected as in Figure 1. (B) The same peptides were spray-coated onto Thermanox coverslips (0.5 μg/cm2) and perfused with whole blood at a shear rate of 300 s–1 for 5 minutes; then surface coverage was measured as described for Figure 1. (C) Peptide no. 23 and peptide GPC-(GPP)5-GPRGQOGVMGFO-(GPP)5-GPC-NH2 were immobilized onto a Biacore CM5 sensor chip via their free cysteine residues. The equilibrium binding capacity of the peptides was determined for different concentrations of plasma-derived VWF. (D) Collagen (100 μg/mL) was immobilized on a 96-well plate or a Thermanox coverslip and incubated with purified VWF (1 μg/mL) or perfused with whole blood in the presence or absence of different concentrations of peptide GPC-(GPP)5-GPRGQOGVMGFO-(GPP)5-GPC-NH2. VWF binding and platelet deposition were evaluated as in Figure 1. Shown is a representative of 3 independent experiments, each performed in duplicate (A), triplicate (B,D), or as single determinations (C). Error bars denote SD.
Identification of the minimal collagen sequence required for VWF binding. (A) The truncated and alanine-modified peptides derived from peptide no. 23 (Table S2) were immobilized on a 96-well plate and incubated with plasma-derived VWF (1 μg/mL), and bound VWF was detected as in Figure 1. (B) The same peptides were spray-coated onto Thermanox coverslips (0.5 μg/cm2) and perfused with whole blood at a shear rate of 300 s–1 for 5 minutes; then surface coverage was measured as described for Figure 1. (C) Peptide no. 23 and peptide GPC-(GPP)5-GPRGQOGVMGFO-(GPP)5-GPC-NH2 were immobilized onto a Biacore CM5 sensor chip via their free cysteine residues. The equilibrium binding capacity of the peptides was determined for different concentrations of plasma-derived VWF. (D) Collagen (100 μg/mL) was immobilized on a 96-well plate or a Thermanox coverslip and incubated with purified VWF (1 μg/mL) or perfused with whole blood in the presence or absence of different concentrations of peptide GPC-(GPP)5-GPRGQOGVMGFO-(GPP)5-GPC-NH2. VWF binding and platelet deposition were evaluated as in Figure 1. Shown is a representative of 3 independent experiments, each performed in duplicate (A), triplicate (B,D), or as single determinations (C). Error bars denote SD.
We previously reported putative VWF-binding sites in collagen III,18 but VWF bound weakly to these synthetic triple-helical peptides compared with full-length collagen. These peptides were short stretches of cyanogen bromide fragments of bovine collagen, and so lacked the intact high-affinity binding site, which was cleaved in the parent collagen by cyanogen bromide digestion at the methionine residue within the sequence that we now report.
The triple-helical peptide GPC-(GPP)5-GPRGQOGVMGFO-(GPP)5-GPC-NH2 at 500 μg/mL (∼ 40 μM) inhibited binding of VWF, or platelets from whole blood, to immobilized collagen III under flow conditions almost completely (Figure 2D).
These results confirm the sequence RGQOGVMGF as the major, high-affinity VWF-binding site and exclude the prominent role for the VWF A1 domain in collagen binding postulated previously.19 We cannot exclude the possibility that the residual binding of the A3-domain mutant, His1023Ala, reflects weak binding of VWF to peptide no. 23 through A1, nor that binding of A3 is required to render A1 competent to bind collagen at a separate site.
Our data suggest that inhibition of VWF binding to collagen, by peptides, antibodies, or other antagonists that target this nonapeptide collagen sequence, may be a useful therapeutic strategy. Current antiplatelet therapy addresses platelet activation through amplification pathways including purinergic receptors and thromboxane generation, or the up-regulation of the fibrinogen receptor integrin αIIbβ3. Previous use of in vivo models suggested that inhibition of the collagen-VWF interaction with a monoclonal antibody or a naturally occurring collagen-binding protein, by targeting primary platelet adhesion to the arterial subendothelium, has a better risk-benefit ratio than inhibition of platelet-platelet interaction,9,10 and might provide a valuable alternative to the antiplatelet therapies in current clinical practice (recently reviewed in Ahrens et al20 ).
The sequence RGQOGVMGF (underlined) occurs in only one other human protein, collagen II. A peptide, GPC-(GPP)5-GAOGEDGROGPOGPQGARGQOGVMGFO-(GPP)5-GPC (amino acids 510-536 from collagen II) bound VWF with high affinity (data not shown). This may be irrelevant to hemostasis, since collagen II is not found in vascular subendothelium, being largely restricted to cartilage and vitreous humor. The sequence RGQOGVMGF is 100% conserved in collagen III from mouse, rat, cow, and chicken. In human collagen I, a heterotrimer comprising 2 α1 and 1 α2 chains, a closely related sequence occurs in the α1 chain, differing by a single amino acid (RGQAGVMGF). This O-to-A substitution prevented the VWF-binding activity in our homotrimeric synthetic peptide from the Ala-scanned set. In this region, the sequence RGQAGVMGF of the human α1(I) chain aligns with the sequence RGEOGNIGF of the α2(I) chain, suggesting that the essential O in position 4 of the VWF-binding homotrimer, although substituted with an A in the α1(I) chain, is provided by position 4 of the α2(I) chain sequence, so that VWF may bind collagens I, II, and III in an identical manner. Modeling experiments, shown in Figure S1, support this conclusion.
Authorship
The authors declare no competing financial interests.
T.L., N.R., P.G.G., and R.W.F. contributed equally to this work.
Prepublished online as Blood First Edition Paper, August 15, 2006; DOI 10.1182/blood-2006-03-011965.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported in part by a grant from the Wellcome Trust.