Abstract
The dynamics of human T-lymphotropic virus type-1 (HTLV-1) provirus expression in vivo are unknown. There is much evidence to suggest that HTLV-1 gene expression is restricted: this restricted gene expression may contribute to HTLV-1 persistence by limiting the ability of the HTLV-1–specific CD8+ cell immune response to clear infected cells. In this study, we tested the hypothesis that derepression of HTLV-1 gene expression would allow an increase in CD8+ cell–mediated lysis of HTLV-1–infected cells. Using histone deacetylase enzyme inhibitors (HDIs) to hyperacetylate histones and increase HTLV-1 gene expression, we found that HDIs doubled Tax expression in naturally infected lymphocytes after overnight culture. However, the rate of CD8+ cell–mediated lysis of Tax-expressing cells ex vivo was halved. HDIs appeared to inhibit the CD8+ cell–mediated lytic process itself, indicating a role for the microtubule-associated HDAC6 enzyme. These observations indicate that HDIs may reduce the efficiency of cytotoxic T-cell (CTL) surveillance of HTLV-1 in vivo. The impact of HDIs on HTLV-1 proviral load in vivo cannot be accurately predicted because of the widespread effects of these drugs on cellular processes; we therefore recommend caution in the use of HDIs in nonmalignant cases of HTLV-1 infection.
Introduction
Human T-lymphotropic virus type 1 (HTLV-1) is a complex retrovirus that preferentially infects CD4+ CD45RO+ T cells in vivo1,2 in 10 to 20 million people worldwide. It causes leukemia or inflammatory disease in about 5% of seropositive individuals. HTLV-1 encodes a number of regulatory proteins,3,4 the most studied of which is Tax. Tax is a pleiotropic transcriptional transactivator and is central to the HTLV-1 life cycle. Tax increases the expression of many cellular genes, including several genes involved in T-cell activation and proliferation.5 Tax expression is thus promitotic and drives CD4+ T-cell proliferation to increase HTLV-1 proviral load.6,7
The host cellular immune response counteracts this Tax-mediated proliferation and increase in proviral load. Tax is also the immunodominant HTLV-1 antigen recognized by the strong CD8+ cytotoxic T-cell (CTL) response.8 Up to 10% of circulating CTLs from an HTLV-1–infected individual can be specific for a single Tax epitope (Tax11-19).9,10 The steady-state HTLV-1 proviral load is therefore likely to be determined by proliferation of infected cells balanced by the clearance of productively infected cells by CD8+ cells.11,12
The in vivo dynamics of HTLV-1 proviral expression are not yet clear. In the peripheral blood very little virus expression has been detected either as mRNA or protein,13-16 but HTLV-1 is spontaneously expressed during short-term culture ex vivo.1 These observations have led to the suggestion that HTLV-1 expression is either absent or severely restricted in vivo, thus contributing to the ability of HTLV-1 to persist by avoiding the immune response.
However, the level of HTLV-1 gene expression in naturally infected peripheral blood mononuclear cells (PBMCs) during short-term culture can be increased if CD8+ cells are removed.1 The immune response to HTLV-1 is large and chronically activated,9,17 suggesting constant production of, and exposure to, viral antigen in vivo. It has therefore also been suggested that the lack of detectable viral antigen in the peripheral blood of an HTLV-1–infected individual is due to efficient immune surveillance in vivo rather than latency of the virus.1,11 It remains equivocal whether HTLV-1 is latent, whether expression is restricted to a few infected cells at any one time, or if expression is active in the majority of infected cells in vivo.
The expression of HTLV-1 and other retroviruses depends on the proviral integration site18 and is subject to epigenetic control mechanisms including histone acetylation and DNA methylation.19-24 The level of histone acetylation is determined by a balance between the activity of 2 enzyme families: the histone acetyl transferases (HATs) and the histone deacetylases (HDACs).25 HAT enzymes acetylate histones, opening chromatin and aiding transcription, whereas HDAC enzymes deacetylate histones and reduce transcription. Inhibition of HDAC activity therefore results in histone hyperacetylation and an increase in gene expression. Tax is known to recruit HAT enzymes to the LTR26 to acetylate local histones, open chromatin structure, and aid viral transcription. In addition, Tax is known to exclude HDAC enzymes from the LTR,27,28 preventing histone deacetylation and closure of active chromatin. These observations suggest that histone acetylation is an important mechanism of HTLV-1 gene expression regulation.
Patients, materials, and methods
Cells, culture, and HDAC inhibitors
HTLV-1–infected PBMCs were isolated by density centrifugation on Histopaque (Sigma, Poole, Dorset, United Kingdom) from EDTA-anticoagulated blood samples taken from HTLV-1–infected individuals (for full patient details, see Table S1, available at the Blood website; see the Supplemental Materials link at the top of the online article). All individuals attended the HTLV-1 clinic at St Mary's Hospital, London. This study was conducted as part of a program of research in this cohort that was approved by St. Mary's Local Research Ethics Committee. All patients gave written informed consent. Isolated PBMCs were washed twice in PBS then cryopreserved in FCS/10% dimethyl sulfoxide until use. When required cells were thawed, washed twice in PBS, placed in complete medium (RPMI-1640, 10% FCS, pen/strep l-glutamine) in 5-mL vented capped tubes, and cultured at 37°C, 5% CO2 for 18 hours. If CD8+ cell–depleted PBMCs were required, CD8+ cells were removed by positive selection using magnetic microbeads following the manufacturer's instructions (Miltenyi Biotec, Bisley, Surrey, United Kingdom). The median CD8+ cell depletion achieved was 93% (range, 84% to 98%). When appropriate, HDIs sodium valproate (NaV), sodium butyrate (NaB), and trichostatin A (TSA; all from Sigma) were added to the culture medium at the required concentration at the start of the culture period. NaV and NaB were used at a final concentration of 5 mM, and TSA was used at a final concentration of 350 nM (optimal concentrations, as determined by dose response curves, Figure S1) unless otherwise stated. Tubacin,31 an HDAC6-specific inhibitor, and its negative control compound niltubacin were used in a similar manner at a final concentration of 10 μM. Niltubacin has a similar biochemical structure to tubacin but does not affect HDAC function.
Detection of Tax expression
To detect Tax expression in HTLV-1–infected cells, either whole PBMCs or CD8+ cell–depleted PBMCs were incubated for 18 hours with or without HDIs as described in the previous paragraph. Cells were then fixed with 2% paraformaldehyde for 20 minutes at room temperature and surface stained with monoclonal antibodies to CD4 and CD8 (each at 15 μg/mL; Beckman Coulter, High Wycombe, United Kingdom) in the presence of 7% normal goat serum (NGS) for 15 minutes at room temperature. Cells were then permeabilized with 0.1% Triton X-100 in PBS for 10 minutes at room temperature. Finally, cells were stained intracellularly with the anti-Tax protein Lt-4-FITC–conjugated antibody32 diluted 1/100 in PBS/7% NGS for 25 minutes at room temperature, and then washed and analyzed on a Coulter Epics XL flow cytometer (Beckman Coulter). Thirty thousand events were routinely collected. Viable lymphocytes were gated for closer analysis using Expo32 analysis software (Beckman Coulter).
Proviral load measurement
DNA was extracted from 2 × 106 PBMCs following the manufacturer's protocol (DNeasy Tissue Kit; Qiagen, Crawley, West Sussex, United Kingdom) and eluted in polymerase chain reaction (PCR) grade H2O. Three dilutions of eluted DNA (1:4, 1:8, 1:16) were amplified for HTLV-1 DNA (Tax-specific primers as in Seiki et al33 and Kwok et al34 ) and β-actin by real-time quantitative PCR in a Roche light cycler (Roche Diagnostics, Lewes, East Sussex, United Kingdom) using SYBR Green 1 Dye incorporation. Incorporation was detected at 85°C at the end of each of 45 amplification cycles. Standard curves were generated using DNA from the TARL-235 cell line, which carries a single HTLV-1 provirus copy per cell. The sample copy number was estimated by interpolation from the standard curve, calculated as an average of the 3 dilutions and expressed as the proportion of PBMCs infected.
Calculation of theoretical maximum Tax expression
To calculate the theoretical maximum frequency of Tax expression in CD4+ cells taken from HTLV-1–infected individuals, we used the following equation: max Tax = (proviral load × 100)/(proportion of PBMCs that are lymphocytes × proportion of lymphocytes that are CD4+). In this calculation, we made the simplifying assumptions that there is one provirus copy per cell and that the proviral load is carried solely in CD4+ cells. The frequency of CD4+ cells and the frequency of lymphocytes in PBMCs were assayed by flow cytometry as previously described.36
CD8+ cell lytic efficiency assay
The rate (or “efficiency”) of CD8+ cell–mediated lysis of HTLV-1–infected cells was estimated as recently described.36 PBMCs were thawed and washed, and CD8+ cells were positively selected (as described in “Cells, culture, and HDAC inhibitors,” above) and titrated back into the CD8-depleted fraction at CD8+/CD8– ratios above, below, and including the physiological ratio for that individual. Cells were then cocultured at 37°C for 18 hours, harvested, and stained for Tax, CD4, and CD8 as described in “Detection of Tax expression,” above. The proportion of Tax+ CD4+ cells surviving coculture was plotted against the proportion of CD8+ cells present and a mathematical model then fitted to the data. CD8+ cell lytic efficiency (expressed as the proportion of Tax-expressing CD4+ cells killed per CD8+ cell per day) was calculated for each HTLV-1–infected individual tested. When required, HDIs were added at the beginning of the coculture period. All assays were done in duplicate and the results are presented as the mean CD8+ cell lytic efficiency.
51Cr release assay of cytotoxicity
Natural killer T (NKT) cells and the T-cell clone 1G4 were pretreated for 18 hours with niltubacin or tubacin31 at 10 μM, or NaV at 5 mM, and then used as effectors in a 4-hour 51Cr release assay. For NKT-cell killing, C1RCD1d target cells were incubated with 51Cr and 1 μM α-galactosylceramide or vehicle solution for 1.5 hours before washing in RPMI and incubating with NKT cells. For 1G4 killing, T2 cells were incubated with 51Cr and 1 μM SLLMWITQV37 or an irrelevant peptide (GILGFVFTL) for 1.5 hours before washing in RPMI and incubating with 1G4 cells. Cells were cultured in triplicate at effector-target (E/T) ratios of 9:1, 3:1, and 1:1. Only viable cells, as determined by trypan blue exclusion, contributed to the E/T ratio. Maximum release was determined by incubating target cells with 5% Triton X-100, and spontaneous release was determined by incubating target cells without effectors. Percent specific lysis was calculated as (sample–minimum)/(maximum–minimum).
Assay of sodium valproate toxicity
PBMCs were cultured in the presence or absence of 5 mM NaV for 18 hours, harvested, and resuspended in PBS/1% FCS supplemented with 20 μg/mL 7-aminoactinomycin D (7-AAD; Sigma) and CD4-PC5, CD8-RD1 antibodies (concentrations as given under “Cells, culture, and HDAC inhibitors,” above; Beckman Coulter). Cells were then incubated at 4°C for 20 minutes, washed once in PBS/1% FCS supplemented with 20 μg/mL actinomycin D (AD; Sigma), and fixed in 2% paraformaldehyde with 10 μg/mL AD for 20 minutes at room temperature. Cells were then washed in PBS, stained intracellularly for Tax if necessary, and analyzed by flow cytometry as described in “Detection of Tax expression,” above.
Statistical analysis
Both parametric and nonparametric statistical tests were used as appropriate, taking the null hypothesis and the sample size into account. The Wilcoxon signed rank test38 was used to determine the significance of the observed change in paired observations for a single parameter with and without HDI treatment across all HTLV-1–infected individuals tested. The Spearman rank-order correlation38 coefficient was calculated when the significance of observed changes in 2 parameters across all HTLV-1–infected individuals was tested. All tests were 2 tailed and rejected or accepted at the 95% level.
Results
Effects of HDAC inhibitors on HTLV-1 gene expression
HDIs increased HTLV-1 gene expression in a dose-dependent manner. It was first necessary to test the effects of 3 HDIs (sodium valproate [NaV], sodium butyrate [NaB], and trichostatin A [TSA]) on the expression level of the HTLV-1 viral protein Tax in whole PBMCs isolated from HTLV-1–infected individuals. In all cases, the proportion of Tax-expressing CD4+ cells increased in a dose-dependent manner over the concentration ranges tested (Figure 1A). NaB and NaV were tested over a therapeutically relevant range (1 to 5 mM), while TSA, a toxic and potent HDI, was tested in the nanomolar range.
We next wished to quantify the increase in Tax expression caused by HDI treatment in the absence of CD8+ cells, which normally reduce Tax expression.1 The results show that each HDI significantly increased Tax expression in CD4+ cells in the absence of CD8+ cells (Wilcoxon signed rank test: P < .001 for NaV, NaB, and TSA) (Figure 1B). On average, Tax expression in CD4+ cells after 18-hour in vitro incubation increased 1.79-fold with NaV treatment, 1.77-fold with NaB, and 2.04-fold with TSA compared with untreated CD4+ cells. Thus, treatment of HTLV-1–infected cells with HDIs, in the absence of CD8+ cells, approximately doubled the proportion of CD4+ cells expressing Tax at 18 hours.
Acetylation controls an estimated 9% of proviral expression.
The mean proportion of provirus+ CD4+ cells that expressed Tax after 18-hour culture was 13% in untreated cells and rose to 22% in HDI-treated cells. This suggested that in vitro approximately 9% of the theoretic maximum Tax expression (as estimated from the proviral load) is regulated by protein acetylation. All 3 HDIs tested gave a similar result (data not shown).
HDI increased Tax expression in HTLV-1–infected cells. (A) HDI increased the frequency of Tax expression in CD4+ cells (cultured as whole PBMCs for 18 hours) in a dose-dependent manner. NaV and NaB concentrations tested were in the millimolar range. TSA concentrations tested were in the nanomolar range. One representative experiment of 6 is shown. (B) HDIs significantly increased the frequency of Tax+ CD4+ cells in the absence of CD8+ cells; * all P < .008 (Wilcoxon signed rank test; 2-tailed). Horizontal bars represent the median frequency of Tax expression.
HDI increased Tax expression in HTLV-1–infected cells. (A) HDI increased the frequency of Tax expression in CD4+ cells (cultured as whole PBMCs for 18 hours) in a dose-dependent manner. NaV and NaB concentrations tested were in the millimolar range. TSA concentrations tested were in the nanomolar range. One representative experiment of 6 is shown. (B) HDIs significantly increased the frequency of Tax+ CD4+ cells in the absence of CD8+ cells; * all P < .008 (Wilcoxon signed rank test; 2-tailed). Horizontal bars represent the median frequency of Tax expression.
Effects of HDAC inhibitors on CD8+ cell–mediated lysis
We wished to test whether the increased Tax expression caused by HDIs led to an increase in CD8+ cell lytic function. We chose to focus on the effects of NaV, because NaV has long been in therapeutic use in humans and is well tolerated.
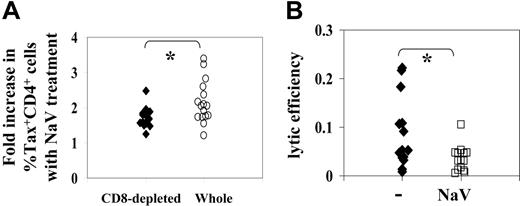
CD8+cell lytic efficiency was halved by treatment with sodium valproate. First, we compared the NaV-mediated increase in the proportion of Tax+ CD4+ cells between whole PBMCs and CD8+ cell–depleted PBMCs cultured ex vivo for 18 hours. Surprisingly, we found (Figure 2A) that the NaV-mediated increase in the frequency of Tax expression was significantly greater (P < .02, Wilcoxon signed rank, 2-tailed test) in whole PBMCs than in CD8+ cell–depleted PBMCs (median fold increase in whole PBMCs: 1.8; median fold increase in depleted PBMCs: 2.07). This observation suggested that NaV increased virus gene expression in CD4+ cells directly but also might impair CD8+ cell activity, allowing Tax expression to increase further.
Using a recently developed ex vivo CD8+ cell killing assay,36 we therefore quantified CD8+ cell lytic efficiency (the proportion of Tax-expressing CD4+ cells killed per average CD8+ cell per day) in the presence and absence of NaV in 14 HTLV-1–infected individuals. Each assay was performed in duplicate and the mean lytic efficiency calculated. CD8+ cell lytic efficiency was on average halved by treatment with NaV (Wilcoxon signed rank test: P = .002) (Figure 2B).
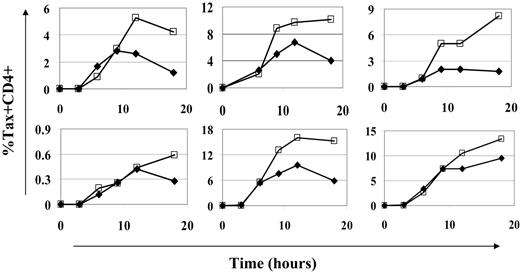
Sodium valproate did not significantly alter the timing of the onset or the peak of Tax expression. If HDI treatment significantly altered the kinetics (ie, the timing of the onset or the peak) of Tax expression in HTLV-1–infected cells, the estimate of CD8+ cell lytic efficiency might be affected. CD8+ cell lytic efficiency is measured at the end of 18-hour culture. Any delay in the onset of Tax expression would reduce the time over which infected cells were exposed to CD8+ cell lysis. This would in turn result in a reduced estimate of CD8+ cell lytic efficiency. Consequently, we examined the time course of Tax expression in CD8+ cell–depleted PBMCs from 6 HTLV-1–infected individuals in the presence and absence of NaV. The peak of Tax expression occurred between 12 and 18 hours after the start of ex vivo culture. Although NaV treatment increased the magnitude of Tax expression, the timing of neither the onset nor the peak of Tax expression was altered by NaV treatment (Figure 3). The largest observed difference in the frequency of Tax expression between NaV-treated and untreated cells was seen during the last 6 hours of culture. Similar results were obtained with TSA treatment (data not shown).
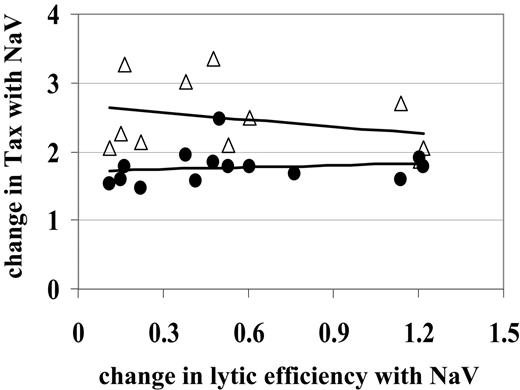
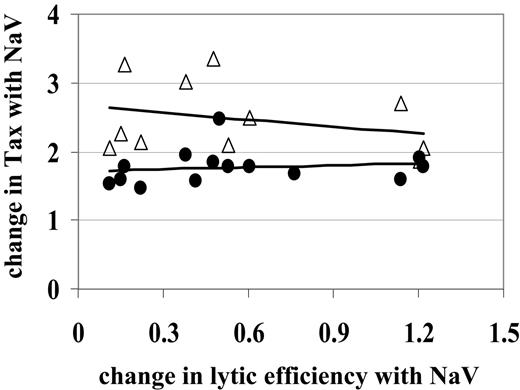
The observed decrease in CD8+cell lytic efficiency was not due to an increase in Tax expression in CD4+or CD8+cells. Since CD8+ cell lytic efficiency is calculated as a proportion of loss of Tax+ CD4+ cells, we hypothesized that the observed decrease in lytic efficiency was in fact due to the increase in Tax-expressing CD4+ cells. For example, if an average CD8+ cell kills only 2 of 100 Tax+ CD4+ cells in the test period, our estimate of CD8+ cell rate of lysis would decrease from 2% (2/100) to 1% (2/200) as the number of Tax-expressing cells was doubled by HDI treatment. However, Figure 4 shows that the change in CD8+ cell lytic efficiency due to NaV treatment did not correlate with the change in Tax expression in CD4+ cells (Spearman rank correlation test, P > .2).
NaV decreases CD8+cell antiviral function. (A) The NaV-mediated fold increase in the % Tax-expressing CD4+ cells was compared in whole PBMCs and CD8+ cell–depleted PBMCs. NaV treatment increased the % Tax+ CD4+ cells to a greater extent (*P < .02, Wilcoxon signed rank, 2-tailed) in whole PBMCs. (B) Ex vivo CD8+ cell lytic efficiency (the proportion of Tax-expressing CD4+ cells killed per CD8+ cell per day) was estimated in 14 HTLV-1–infected individuals with or without NaV treatment. NaV was found to significantly decrease lytic efficiency (*P = .002).
NaV decreases CD8+cell antiviral function. (A) The NaV-mediated fold increase in the % Tax-expressing CD4+ cells was compared in whole PBMCs and CD8+ cell–depleted PBMCs. NaV treatment increased the % Tax+ CD4+ cells to a greater extent (*P < .02, Wilcoxon signed rank, 2-tailed) in whole PBMCs. (B) Ex vivo CD8+ cell lytic efficiency (the proportion of Tax-expressing CD4+ cells killed per CD8+ cell per day) was estimated in 14 HTLV-1–infected individuals with or without NaV treatment. NaV was found to significantly decrease lytic efficiency (*P = .002).
While the majority of the HTLV-1 proviral load is carried by the CD4+ T-cell population, a detectable proportion is carried by CD8+ T cells.39,40 HDIs increased Tax expression in CD8+ cells as well as in CD4+ cells. In fact, the NaV-mediated increase in Tax expression in CD8+ cells (on average, a 2.48-fold increase) was significantly greater (Wilcoxon rank sum test, P = .001) than the increase seen in CD4+ cells (on average, a 1.73-fold increase). We therefore tested the hypothesis that the increase in Tax expression in CD8+ cells with NaV treatment resulted in the observed decrease in lytic efficiency via increased fratricide39 or functional impairment41,42 of the CD8+ cells. We found that the change in lytic efficiency due to NaV treatment did not correlate with the change in Tax expression in CD8+ cells (Figure 4; Spearman rank correlation test, P > .2). We conclude that NaV reduces CD8+ cell–mediated lysis by acting directly on the effector cells, not by activating HTLV-1 expression in CD8+ or CD4+ cells.
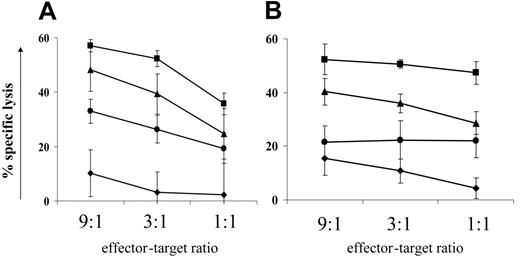
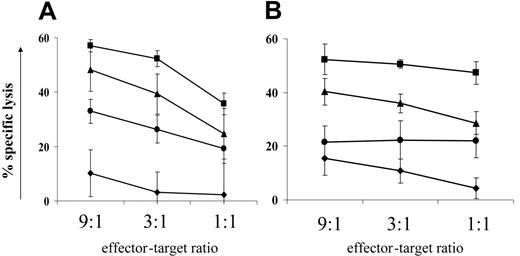
Sodium valproate decreased target cell lysis in an independent system. To test the possibility that NaV directly affected the ability of the CD8+ cells to lyse target cells independently of HTLV-1 infection, a human CTL cell line (clone 1G4) specific for the tumor antigen NY-ESO37 was pretreated with NaV for 18 hours and then used in 51Cr release assays against antigen-pulsed target cells. We found that (Figure 5A) NaV reduced specific lysis by 37% (mean of duplicates: x1 = 30%, x2 = 44%) (calculated at maximal killing comparing NaV treatment with negative control treatment). A similar reduction in target cell killing was also seen with a CD4+ perforin+ cytotoxic NKT-cell line against α-galactosylceramide-loaded targets (Figure 5B). We therefore concluded that NaV treatment directly impairs CTL function.
HDI treatment did not alter the kinetics of Tax expression. CD8+ cell–depleted PBMCs were cultured for 18 hours with (□) or without (♦) NaV, and Tax expression was assessed at intervals. Onset of Tax expression was not affected by NaV treatment. NaV had its greatest effect on the level of Tax expression reached, especially in the latter half of the culture period. Each graph represents cells from a different HTLV-1–infected individual.
HDI treatment did not alter the kinetics of Tax expression. CD8+ cell–depleted PBMCs were cultured for 18 hours with (□) or without (♦) NaV, and Tax expression was assessed at intervals. Onset of Tax expression was not affected by NaV treatment. NaV had its greatest effect on the level of Tax expression reached, especially in the latter half of the culture period. Each graph represents cells from a different HTLV-1–infected individual.
NaV decreases CD8+cell lytic efficiency independently of the increase in Tax expression. The observed decrease in lytic efficiency (where the change calculated by lytic efficiency with NaV/lytic efficiency was < 1) did not correlate with the observed increase in Tax expression (where Tax with NaV/Tax is > 1) in either CD4+ (•) or CD8+ (▵) cells. Straight lines are lines of best fit to aid visual interpretation of the data.
NaV decreases CD8+cell lytic efficiency independently of the increase in Tax expression. The observed decrease in lytic efficiency (where the change calculated by lytic efficiency with NaV/lytic efficiency was < 1) did not correlate with the observed increase in Tax expression (where Tax with NaV/Tax is > 1) in either CD4+ (•) or CD8+ (▵) cells. Straight lines are lines of best fit to aid visual interpretation of the data.
An HDAC6-specific inhibitor reduces cell-mediated cytotoxicity. The data presented in the previous paragraph and in the section describing the halving of CD8+ cell lytic efficiency showed that NaV treatment significantly decreased the lytic effector function of cytotoxic lymphocytes in 3 independent systems. We hypothesized that NaV inhibited the function of deacetylase enzymes with roles other than histone deacetylation. The HDAC family of enzymes has 11 members,43 one of which, HDAC6, is exclusively cytoplasmic and is known to be microtubule associated.44 We used an HDAC6-specific inhibitor, tubacin,31,45 to test whether HDAC6 played a role in cell-mediated cytotoxicity. Tubacin inhibits α-tubulin deacetylation in long-term cell lines in vitro, but has no detectable effect in PBMCs.45,46 We therefore pretreated the 1G4 CTL line and 2 different NKT cell lines with tubacin, or the negative control compound niltubacin, for 18 hours before adding them to their respective 51Cr-loaded target cells. One experiment was carried out with the 1G4 line, and 2 experiments each with the 2 NKT cell lines. Representative results are shown in Figure 5: in all 3 cell lines, specific lysis was reduced by approximately 20% (1G4 cell line: 16%; NKT cell lines: mean = 23%) at the top E/T ratio. We conclude that HDAC6 does indeed play a role in cell-mediated cytotoxicity and that its inhibition may contribute to the reduction in killing seen with the broad-spectrum HDI NaV.
HDIs directly reduce cell-mediated cytotoxicity in an independent system, partly by inhibiting HDAC6.51Cr release assays were used to test the effect of 2 HDIs (NaV and the HDAC6-specific inhibitor tubacin) on cell-mediated cytotoxicity. (A) An NY-ESO–specific CTL cell line (1G4) was cocultured with peptide-loaded target cells after 18-hours pretreatment with HDIs. (B) A CD1d-restricted NKT-cell line was cocultured with α-galactosylceramide–loaded target cells after 18-hours pretreatment with HDIs. ▪ indicate negative control treatment; •, sodium valproate; ▴, tubacin treatment; and ♦, no antigen loaded onto target cells. Treatment with either NaV or tubacin decreased specific lysis at maximal killing by approximately 40% and 20%, respectively. Data plotted are the mean of triplicate measures for one experiment ± 1 SD.
HDIs directly reduce cell-mediated cytotoxicity in an independent system, partly by inhibiting HDAC6.51Cr release assays were used to test the effect of 2 HDIs (NaV and the HDAC6-specific inhibitor tubacin) on cell-mediated cytotoxicity. (A) An NY-ESO–specific CTL cell line (1G4) was cocultured with peptide-loaded target cells after 18-hours pretreatment with HDIs. (B) A CD1d-restricted NKT-cell line was cocultured with α-galactosylceramide–loaded target cells after 18-hours pretreatment with HDIs. ▪ indicate negative control treatment; •, sodium valproate; ▴, tubacin treatment; and ♦, no antigen loaded onto target cells. Treatment with either NaV or tubacin decreased specific lysis at maximal killing by approximately 40% and 20%, respectively. Data plotted are the mean of triplicate measures for one experiment ± 1 SD.
Sodium valproate treatment was mildly toxic to lymphocytes from HTLV-1–infected individuals. To investigate the contribution of direct NaV toxicity to the observed decrease in CD8+ cell lytic efficiency, we assayed NaV-treated, HTLV-1–infected PBMCs for uptake of 7-AAD. 7-AAD is a DNA intercalating dye that is taken up by cells with damaged membranes (including apoptotic cells) and can be used to assess cell viability.47,48 7-AAD was chosen over annexinV/propidium iodide (PI) staining because Tax staining occupied the fluorescence channel needed for annexinV. 7-AAD staining offers equivalent information to annexinV/PI staining on cell viability while occupying only a single fluorescence channel.
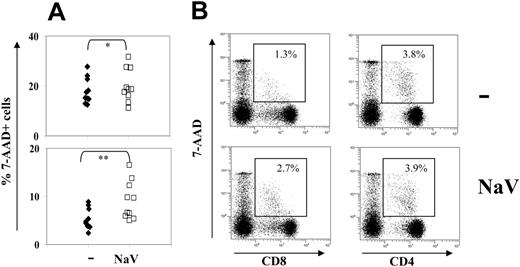
NaV treatment did not cause a significant change in the forward versus side scatter plots of PBMCs, suggesting that there was no increase in apoptotic bodies or cell debris. However, NaV treatment was found (Figure 6A) to significantly increase 7-AAD uptake by lymphocytes (Wilcoxon signed rank, P = .014), in particular CD8+ cells (P = .002) but not CD4+ cells (P = .195; Figure 6B). This finding suggested that NaV was preferentially toxic toward CD8+ cells.
Toxicity of sodium valproate in lymphocytes does not contribute to the observed reduction in CD8+cell lytic efficiency. The increase in 7-AAD uptake by CD8+ cells following treatment with NaV raised the question whether the observed decrease in CD8+ cell–mediated lysis was caused by nonspecific toxicity. To test this, we excluded 7-AAD+ CD8+ cells from the analysis and calculated the lytic efficiency of the remaining 7-AAD– CD8+ cells. The results showed that addition of NaV still halved the lytic efficiency of the 7-AAD– CD8+ cells. Furthermore, there was no significant correlation between the reduction in lytic efficiency and NaV-induced uptake of 7-AAD in either CD4+ or CD8+ cells (Spearman rank correlation, P > .1 [CD8+], P > .5 [CD4+]).
Discussion
Restricted or delayed proviral transcription may contribute to the persistence of retroviruses in vivo, even in cases—such as HIV-1—where there is high-level persistent infectious replication of the virus. Unexpressed proviruses contribute to the difficulty of clearing retroviral infection with effective antiretroviral drugs. However, the mechanisms of maintenance of transcriptional inactivity in these silent or reactivatable proviruses are unknown. The most likely mechanisms are integration into a transcriptionally inactive region of the genome or epigenetic modifications such as DNA methylation or histone deacetylation. Indeed, recent evidence20,49 suggests that histone deacetylation is responsible for maintaining latency of a significant proportion of the residual HIV-1 genomes.
NaV treatment selectively caused membrane damage in CD8+cells. Whole PBMCs were cultured for 18 hours with or without 5 mM NaV, and stained for CD4, CD8, and 7-AAD uptake. (A) NaV treatment significantly increased 7-AAD uptake by lymphocytes (top panel; *P = .014, Wilcoxon signed rank test) and CD8+ cells (bottom panel; **P = .002, Wilcoxon signed rank test). (B) 7-AAD uptake by CD4+ cells did not increase with NaV treatment (right panels) but only 7-AADlow uptake, associated with early apoptosis, increased in CD8+ cells (left panels). (Top panels) Untreated cells. (Bottom panels) Cells treated with NaV. One representative experiment of 15 is shown.
NaV treatment selectively caused membrane damage in CD8+cells. Whole PBMCs were cultured for 18 hours with or without 5 mM NaV, and stained for CD4, CD8, and 7-AAD uptake. (A) NaV treatment significantly increased 7-AAD uptake by lymphocytes (top panel; *P = .014, Wilcoxon signed rank test) and CD8+ cells (bottom panel; **P = .002, Wilcoxon signed rank test). (B) 7-AAD uptake by CD4+ cells did not increase with NaV treatment (right panels) but only 7-AADlow uptake, associated with early apoptosis, increased in CD8+ cells (left panels). (Top panels) Untreated cells. (Bottom panels) Cells treated with NaV. One representative experiment of 15 is shown.
In this study, we used HDAC inhibitors (HDIs) to derepress HTLV-1 gene expression (as measured by an approximate doubling in Tax expression) and to test the hypothesis that the consequent increase in antigen expression enabled CD8+ cells to lyse HTLV-1–infected cells more efficiently in an ex vivo assay.
We calculated that approximately 13% of the possible maximum provirus+ cells expressed Tax spontaneously during short-term ex vivo culture. The addition of HDIs increased this proportion of Tax-expressing provirus+ cells to 22%, suggesting that acetylation regulates HTLV-1 transcription in approximately 9% of provirus+ cells. This HDI-mediated regulation of provirus expression may be direct or indirect, since it is known that HDIs have a broad spectrum of effects on both gene expression and protein function. If we assume that the ex vivo kinetics of Tax expression reflect in vivo kinetics, our results suggest that only a small fraction of infected cells actively express HTLV-1 proteins at any one time. An increase in frequency of Tax expression from 13% to 22% of provirus+ cells, independently of the exact mechanism, may be highly significant biologically, both by increasing CTL target cell availability and by stimulating the host immune response and lymphocyte proliferation; a small increase in Tax expression will have a significant cumulative effect on proviral load in vivo over time.
We next asked if this restricted virus expression could limit CTL surveillance, recognition, and control of infected cells. To answer this question, we used a newly developed ex vivo CD8+ cell killing assay36 to compare CD8+ cell lytic efficiency in the presence and absence of HDIs, specifically NaV. We found that despite the increased expression of the dominant CTL target antigen Tax, CD8+ cell lytic efficiency was in fact halved by the addition of NaV to the culture medium. The question was then, how does NaV decrease CD8+ cell lytic efficiency?
We considered 3 possible explanations. First, the observed increase in Tax expression after NaV treatment might reduce CD8+ cell lytic efficiency by causing activation-induced cell death in CD8+ T cells. The increased number of Tax-expressing cells might exceed the capacity of CD8+ cells to kill within 18 hours, and so decrease estimates of CD8+ cell lytic efficiency. However, no correlation was found between the observed increase in Tax expression in CD4+ or CD8+ cells and the observed changes in CD8+ cell lytic efficiency with NaV treatment. We therefore concluded that the observed decrease in CD8+ cell lytic efficiency with NaV treatment was not due to the observed increase in the Tax+ CD4+ target cell population.
Two further possibilities remained. First, NaV treatment could directly inhibit CD8+ cell lytic function, and second, NaV treatment could be toxic to CD8+ cells. NaV treatment could inhibit CD8+ cell lytic function by interfering with lytic protein synthesis (and thus granule refilling) or by interfering with the delivery of lytic granules to the site of contact with the target cell. To test this, we used 2 different cytotoxic cell lines (an NY-ESO–specific human CTL cell line and a CD1d-restricted NKT cell line) in 51Cr release assays. In each case, we found that 18 hours of NaV pretreatment reduced the percent specific lysis of target cells by approximately 40%. This observation supported the conclusion that NaV directly impaired CTL-mediated lysis in NaV-treated HTLV-1–infected PBMCs. The observed effect on CTL function was independent of any effects of NaV on CD8+ cell viability, because only viable effector cells contributed to E/T ratios.
Finally, we formally tested the contribution of NaV-induced cell damage to the observed decrease in CD8+ cell lytic efficiency in the HTLV-1 ex vivo assay system. HDIs are known to be proapoptotic, especially toward transformed cells or cell lines,50,51 and are indeed being extensively investigated as potential treatments against cancer.52 Achachi et al53 have recently shown that treatment of bovine leukemia virus (BLV)–infected sheep with NaV significantly reduced their leukemia/lymphoma by triggering apoptosis of infected, transformed B cells. However, NaV has also been used at high doses for the treatment of epilepsy and other neurologic disorders in humans with minimal side-effects. In our study, NaV treatment did not change the forward versus side scatter profiles of HTLV-1–infected PBMCs. This suggested that NaV treatment had no immediate, proapoptotic effect. To quantify the toxicity of and cell damage caused by NaV, we assayed 7-AAD uptake in NaV-treated and untreated cells. We found a significant increase in 7-AAD uptake after NaV treatment by all lymphocytes; this observation held for the CD8+ population but not for the CD4+ population, suggesting that NaV treatment was preferentially toxic toward the CD8+ cells. No significant correlation, however, was found between the increase in 7-AAD uptake by lymphocytes and the decrease in CD8+ cell lytic efficiency with NaV treatment.
A variety of proteins other than histones (eg, transcription factors, α-tubulin) are regulated by HDAC-mediated acetylation.45,54,55 The HDAC enzymes themselves are a large and diverse family.43 One exclusively cytoplasmic HDAC enzyme, HDAC6, is known to be associated with microtubules,44 and there is some suggestion that HDAC6 is associated with the immunologic synapse.56 In addition, CTLs are highly motile cells and require a dynamic cytoskeleton to actively approach potential target cells: inhibition of HDAC6 is known to reduce cell migration.57 We thus proposed that inhibition of HDAC6 was responsible for the observed inhibition of cytotoxic function. To test this theory, we used an HDAC6-specific inhibitor, tubacin,31,45 which inhibits α-tubulin acetylation, but has no effect on nuclear HDACs or transcription. Ideally, we wished to use tubacin in our ex vivo lytic efficiency assay, but preliminary data suggested no effect and tubacin is known to have no effect on tubulin acetylation in PBMCs.45,46 The reasons for this are unknown. However, tubacin significantly alters α-tubulin acetylation in cell lines so we tested tubacin in a 51Cr release cytotoxicity assay. We found that, for the CTL and NKT cell lines tested, 18-hour pretreatment with tubacin reduced specific lysis by about 20%. We therefore concluded that a significant proportion of the observed NaV-mediated inhibition of CD8+ cell lytic efficiency could be explained by inhibition of HDAC6, which is already known to be inhibited by the broad-spectrum HDI TSA. To our knowledge, this is the first time that a functional role for HDAC6 in cell-mediated cytotoxicity has been shown. It will be interesting to pursue the molecular mechanism behind this inhibition and to dissect more fully which part of the cytotoxic pathway is affected.
While in vivo treatment of leukemic BLV-infected sheep with NaV has been shown53 to be beneficial we recommend caution in the use of HDIs as a treatment for HTLV-1–related inflammatory diseases. The proapoptotic activity of HDIs may prove effective against transformed cells in HTLV-1–associated adult T-cell leukemia, but care should be taken in those patients whose leukemic CD4+ cells are still capable of expressing Tax and other viral proteins. Since HDI treatment increases Tax expression, this may also increase Tax-driven mitosis in cells already exhibiting aberrant proliferation, while simultaneously reducing the ability of the immune response to control infection. Similarly, in the HTLV-1–associated inflammatory disease (HAM/TSP) HDI treatment is likely to disturb the equilibrium between HTLV-1 and the immune system and may result in an increased proviral load with decreased immune control. Also, an NaV-induced increase in HTLV-1 antigen load may exacerbate HAM/TSP disease as CD8+ cells produce the inflammatory cytokine IFN-γ in response to higher levels of antigen.58,59 However, our study was based on 18-hour short-term ex vivo and in vitro assays focusing on the effects of HDIs on CD4+ and CD8+ cells in isolation. Our results do not preclude possible longer term beneficial effects of HDI treatment in HTLV-1–infected individuals, as in vivo virus-host dynamics are complex and difficult to predict, especially given the widespread effects of HDIs on gene expression and cell activity.
Authorship
A.J.M. designed the study, performed experiments, interpreted data, and wrote the paper; K.N.M. performed experiments and contributed to paper writing; C.M. and D.S. performed experiments; V.C. contributed to experimental design; R.M. and Y.T. provided essential reagents; G.P.T. performed clinical assessment and recruitment of patients with HTLV-1 infection and contributed to the study design; and C.R.B. designed the study, interpreted data, and contributed to paper writing.
The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 15, 2006; DOI 10.1182/blood-2006-03-013235.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
The authors wish to thank Becca Asquith, Adrian Heaps, Mathew Parker, and Mohamed Nejmeddine for careful reading of the paper and interesting discussions. We also gratefully acknowledge the contribution of Stuart Schreiber to this project.
This work was supported by the Leukemia Research Fund, Wellcome Trust, the Initiative for Chemical Genetics, and the Howard Hughes Medical Institute.