Abstract
Cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) maintains peripheral tolerance by suppressing T-cell activation and proliferation but its precise role in vivo remains unclear. We sought to elucidate the impact of CTLA-4 expression on self/tumor-reactive CD8+ T cells by using the glycoprotein (gp) 100–specific T-cell receptor (TCR) transgenic mouse, pmel-1. pmel-1 CLTA-4–/– mice developed profound, accelerated autoimmune vitiligo. This enhanced autoimmunity was associated with a small but highly activated CD8+ T-cell population and large numbers of CD4+ T cells not expressing the transgenic TCR. Adoptive transfer of pmel-1 CLTA-4–/– CD8+ T cells did not mediate superior antitumor immunity in the settings of either large established tumors or tumor challenge, suggesting that the mere absence of CTLA-4–mediated inhibition on CD8+ T cells did not directly promote enhancement of their effector functions. Removal of CD4+ T cells by crossing the pmel-1 CLTA-4–/– mouse onto a Rag-1–/– background resulted in the complete abrogation of CD8+ T-cell activation and autoimmune manifestations. The effects of CD4+ CLTA-4–/– T cells were dependent on the absence of CTLA-4 on CD8+ T cells. These results indicated that CD8+ CLTA-4–/– T-cell–mediated autoimmunity and tumor immunity required CD4+ T cells in which the function was dysregulated by the absence of CTLA-4–mediated negative costimulation.
Introduction
Cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) is an immunomodulatory molecule, expressed on both CD8+ and CD4+ T cells.1-3 By decreasing T-cell responsiveness and raising the threshold for T-cell activation, CTLA-4 plays a critical role in the maintenance of peripheral T-cell tolerance.2,4,5 Mice genetically deficient in CTLA-4 develop profound autoimmunity and die of a lymphoproliferative disease at 4 weeks of age.6-8 Similarly, in humans, CTLA-4 gene polymorphisms have been linked to the development of various autoimmune diseases, including autoimmune hypothyroidism and type 1 diabetes.9
Blockade of CTLA-4 function provides a compelling means of enhancing antitumor immunity since tumors primarily express nonmutated self-antigens.10 Administration of CTLA-4–blocking antibodies has been shown to induce tumor regression in several murine models.11-15 More significantly, recent translation of these findings in clinical trials demonstrated that the administration of a CTLA-4–blocking antibody mediates objective responses in approximately 15% to 20% of patients with metastatic melanoma.16,17 Objective responses correlated with grades III/IV autoimmune manifestations, including dermatitis, enterocolitis, hepatitis, and hypophysitis.17 However, recent evidence using a knock-in humanized CTLA-4 transgenic mouse model indicates that cancer immunity can occur also in the absence of autoimmune disease.18
Although CTLA-4 blockade has been shown to increase autoimmunity and antitumor immunity, the mechanisms underlying these effects remain controversial. Indeed, various models have been proposed identifying the factors mediating CTLA-4–dependent peripheral tolerance.8,19-27 In order to elucidate the mechanisms by which the absence of CTLA-4 signaling results in autoimmunity and enhanced antitumor immunity, we crossed CTLA-4–/– mice onto a self/tumor-reactive CD8+ T-cell receptor (TCR) transgenic (pmel-1) background.28,29 We found that genetic removal of CTLA-4 induced activation and acquisition of effector functions in pmel-1 CD8+ T cells, resulting in the rapid development of severe autoimmune vitiligo. These effects were abrogated by the removal of the CD4+ T-cell population. Therefore, we propose that the lack of CTLA-4 on self/tumor antigen–specific CD8+ T cells results in autoimmunity and tumor immunity only in the presence of nonspecific, dysregulated CD4+ CTLA-4–/– T cells.
Materials and methods
Mice and tumor lines
pmel-1 TCR transgenic mice28 were crossed with CTLA-4–/– mice (kindly provided by James P. Allison, Memorial Sloan Kettering, New York, NY) to derive pmel-1 CTLA-4–/– mice. pmel-1 CTLA-4–/– mice were crossed with Rag-1–/– (Jackson Laboratories, Bar Harbor, ME) mice to derive pmel-1 Rag–/– CTLA-4–/– mice. Female C57BL/6 mice (Jackson Laboratories) at 6 to 12 weeks of age were used as recipients in adoptive transfer experiments. All animal experiments were conducted with the approval of the National Cancer Institute (NCI) Animal Use and Care Committee. B16-F10 (H-2b), a spontaneous glycoprotein (gp) 100+ murine melanoma, was maintained in culture media.28
Adoptive cell transfer, vaccination, and cytokine administration
Mice (6-12 weeks of age) were injected subcutaneously with 2 × 105 to 5 × 105 B16-F10 melanoma cells. Mice (n = 5 for all groups) were treated 10 to 14 days later with intravenous adoptive transfer of pmel-1 CTLA-4+/+ or pmel-1 CTLA-4–/– in vitro–activated splenocytes. Mice were vaccinated with 2 × 107 plaque forming units (PFU) of a recombinant fowlpox virus expressing human gp100 (rFPhgp100; Therion Biologics, Cambridge, MA).28 Recombinant human interleukin-2 (rhIL-2; Chiron, Emeryville, CA) was administered by intraperitoneal injection twice daily at 36 μg/dose, for a total of 6 doses. Preventative experiments were performed as follows: 6to 12-week old mice received 5 Gy of total body irradiation and 1 × 106 cultured pmel-1 CTLA-4+/+ or pmel-1 CTLA-4–/– T cells. The following day, mice were challenged with 5 × 105 B16 cells. Tumors were measured using calipers, and the products of the perpendicular diameters were recorded. All experiments were performed in a blinded, randomized fashion and performed independently at least twice with similar results.
Generation of bone marrow mixed chimeras
Bone marrow (BM) cells were obtained from the femurs and tibias of wild-type (WT), CTLA-4+/+ pmel-1, or CTLA-4–/– C57BL/6 mice. C57BL/6 recipients were irradiated with 10 Gy and injected intravenously with a 1:1 mix of BM cells from different donors as indicated. Chimeras were blindly evaluated for the development of vitiligo at 8 weeks.
Flow cytometry and antibodies
Cells were labeled with monoclonal antibodies (mAbs; BD Biosciences, Pharmingen, San Diego, CA) against Vβ13-FITC (MR12-3), CD8α-APC (53-6.7), CD44-PE (IM7), and CD4-PE (H129.19). Samples were analyzed using a FACSCalibur flow cytometer and CELLquest software (BD Biosciences, San Diego, CA).
In vitro activation and cytokine release
pmel-1 splenocytes were isolated from pmel-1 and pmel-1 CTLA-4–/– mice as described previously.28 Splenocytes were depleted of non-CD8+ T cells using a magnetic-activated cell-sorting (MACS) negative selection column (Miltenyi Biotech, Auburn, CA), and cocultured overnight with 0.1 μM hgp10025-33 pulsed or unpulsed irradiated T-cell–depleted splenocytes (Miltenyi Biotech). Supernatants were analyzed using a mouse interferon γ (IFNγ) enzyme-linked immunosorbent assay (ELISA) kit (Pierce Endogen, Rockford, IL).
Enumeration of CD8+ T cells
Splenocytes from pmel-1 and pmel-1 CTLA-4–/– mice were quantified with trypan blue exclusion and were analyzed by flow cytometry for CD8+ expression by cells. The absolute number of pmel-1 cells per microliter was calculated by multiplying the splenocyte count by the ratio of CD8+/lymphocyte gate.
Statistical analysis
The correlation between genotype and incidence of vitiligo was analyzed with the 2-tailed Fisher exact test. IFNγ release and CD8+ T-cell quantification were analyzed using analysis of variance (ANOVA). Tumor growth curves were compared using the Wilcoxon rank-sum test.
Results
Pmel-1 CTLA-4–/– mice develop autoimmune vitiligo at an accelerated rate
In order to study the effects of the absence of the CTLA-4 molecule on peripheral T lymphocytes in a self-reactive class I–restricted TCR transgenic setting, we crossed pmel-1 mice with CTLA-4–/– mice to obtain pmel-1 CTLA-4–/– mice. While CTLA-4–/– mice have consistently been shown to die between 3 to 4 weeks of age,6-8 crossing these mice onto a pmel-1 background increased their survival to approximately 10 to 12 weeks of age. Furthermore, these mice developed pronounced vitiligo at an accelerated rate compared with littermate controls (Figure 1). At 4 to 6 weeks of age, 227 pups were examined in a blinded fashion for the development of coat depigmentation. A group of mice (49) developed vitiligo over 75% of their body surface (Table 1). Genotypic analysis indicated that 48 (98%) of 49 mice were pmel-1 CTLA-4–/– mice. Development of vitiligo was dependent on both the expression of the Pmel1 transgene and the lack of cell-surface CTLA-4 expression. Autoimmune vitiligo was seen only in 1 of 6 CTLA-4–/– mice (P < .001), and in none of the mice that were heterozygous for the Ctla4 gene (P < .001) (Table 1). Thus, the lack of CTLA-4 expression in the setting of the pmel-1 self/tumorreactive TCR transgenic mouse results in enhanced reactivity against gp100-expressing melanocytes, as manifested by the rapid development of autoimmune vitiligo.
Qualitative changes in pmel-1 CD8+ CTLA-4–/– T cells affect autoimmunity
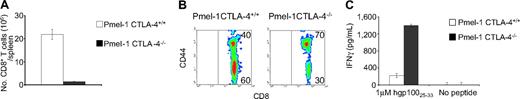
To determine whether the rapid development of vitiligo was due to increased numbers of self/tumor-reactive CD8+ T cells in pmel-1 CTLA-4–/– compared with pmel-1 CTLA-4+/+ mice, we quantified the numbers of splenic CD8+ T cells in these mice. Surprisingly, we found that the number of CD8+ T cells in pmel-1 CTLA-4–/– mice that developed pronounced rapid-onset vitiligo was significantly decreased compared with their pmel-1 CTLA-4 intact littermates (21.9 × 106 ± 2.17 × 106 vs 1.4 × 106 ± 0.13 × 106; P < .001) (Figure 2A). Interestingly, the activation state of the CD8+ T cells from the pmel-1 CTLA-4–/– mice was enhanced compared with that of the pmel-1 CTLA-4+/+ mice. Pmel-1 CTLA-4–/– CD8+ T cells strongly increased the surface expression of activation markers, including CD44 (Figure 2B). Furthermore, the effector functions, as measured by IFNγ release upon coculture with cognate antigen-pulsed target cells, were found to be elevated in pmel-1 CTLA-4–/– CD8+ T cells (Figure 2C). In contrast, as we have previously reported28,30,31 that pmel-1 CD8+ T cells showed a naive phenotype and released minimal amounts of IFNγ in response to the antigen (Figure 2B-C). These findings suggest that qualitative differences rather than quantitative differences in the CD8+ T-cell compartment resulted in the enhanced effector functions in pmel-1 CTLA-4–/– mice and the development of autoimmune vitiligo.
Pmel-1 CTLA-4–/– mice develop autoimmune vitiligo at an accelerated rate compared with pmel-1 CTLA-4+/+ mice. Development of autoimmune vitiligo in a pmel-1 CTLA-4–/– mouse. Pictured are pmel-1 CTLA+/+ (right) and pmel-1 CTLA-4–/– (left) littermates at 6 weeks of age. Autoimmune vitiligo is present over more than 75% of the body surface of the pmel-1 CTLA-4–/– mouse.
Pmel-1 CTLA-4–/– mice develop autoimmune vitiligo at an accelerated rate compared with pmel-1 CTLA-4+/+ mice. Development of autoimmune vitiligo in a pmel-1 CTLA-4–/– mouse. Pictured are pmel-1 CTLA+/+ (right) and pmel-1 CTLA-4–/– (left) littermates at 6 weeks of age. Autoimmune vitiligo is present over more than 75% of the body surface of the pmel-1 CTLA-4–/– mouse.
Absence of CTLA-4 expression on the surface of CD8+ T cells does not enhance their antitumor activity
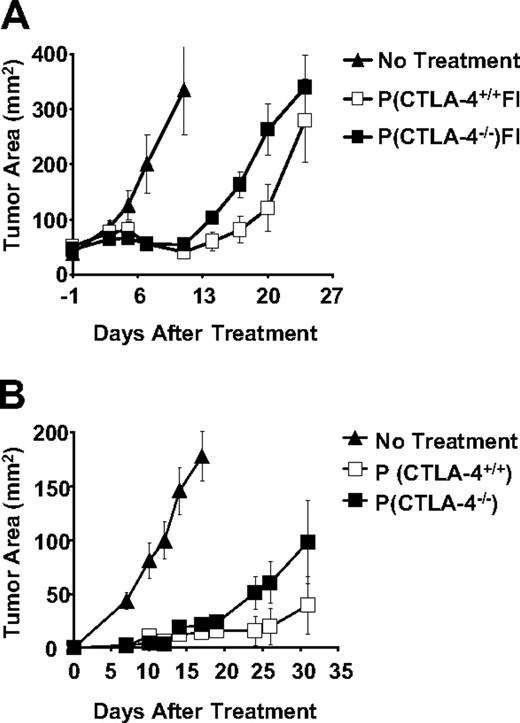
In order to dissect the differential effects of the lack of CTLA-4 expression on CD8+ and CD4+ T-cell subsets, we evaluated the antitumor efficacy of CD8+ T cells from either pmel-1 CTLA-4+/+ or pmel-1 CTLA-4–/– mice upon adoptive transfer. To open a treatment window, we intentionally administered suboptimal doses of pmel-1 cells (1 × 106). As previously described,32,33 1-week-old in vitro–cultured CD8+ T cells were transferred, in combination with rFPhgp100 vaccination and exogenous IL-2, into tumorbearing hosts. Surprisingly, despite the lack of CTLA-4–mediated inhibitory signals, CD8+ T cells from the pmel-1 CTLA-4–/– mice did not result in an improved antitumor response compared with pmel-1 CTLA-4+/+ CD8+ T cells (P = .251) (Figure 3).
Experiments using CTLA-4–blocking antibody have been shown to stimulate productive antitumor immune responses in preventative settings but not in the treatment of large established tumors.34,35 We sought to understand whether our findings were dependent on the read-out system used or whether the lack of CTLA-4 expression on CD8+ T cells was not sufficient to enhance their antitumor activity. Sublethally irradiated C57BL/6 mice received either pmel-1 CTLA-4+/+ or pmel-1 CTLA-4–/– CD8+ T cells and were challenged with B16 tumors after 24 hours. Surprisingly, pmel-1 CTLA-4–/– cells were not more effective than pmel-1 CTLA-4+/+ CD8+ T cells, even when used to protect mice from B16 challenge (P = .297). Therefore, the mere lack of CTLA-4 expression on CD8+ T cells is not sufficient to enhance their effector functions and antitumor activity.
Enhanced pmel-1 CD8+ T-cell function in CTLA-4–/– mice is dependent on CD4+ T cells
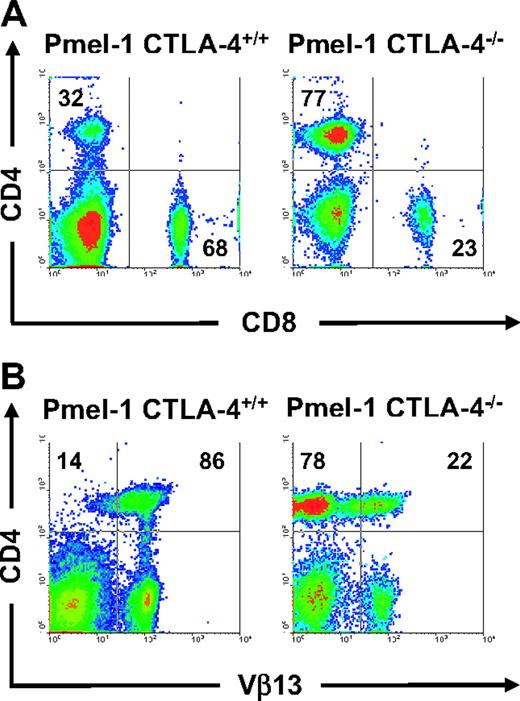
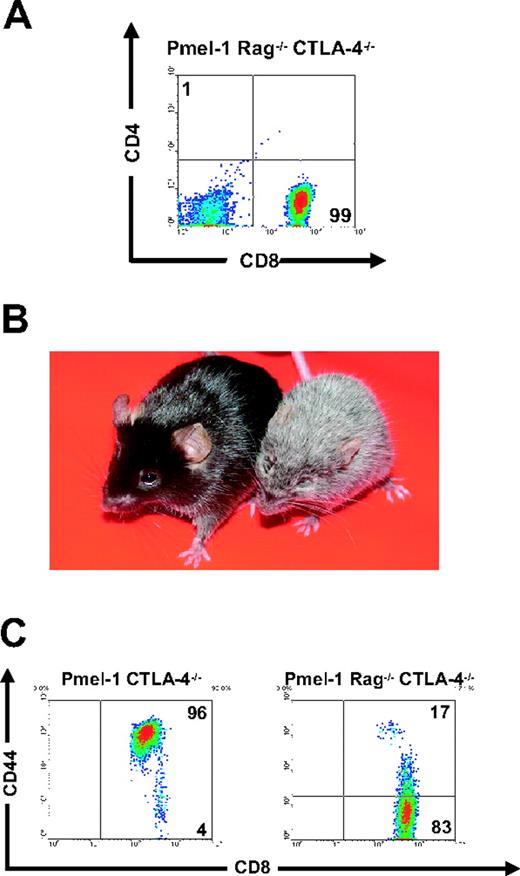
Because we found that the absence of CTLA-4 does not have a direct impact on CD8+ T-cell effector functions, we sought to further delineate the mechanisms underlying CTLA-4–mediated peripheral tolerance. About 70% of the T cells in pmel-1 mice are self/tumor-reactive CD8+ T cells (Figure 4A). In sharp contrast, phenotypic analyses of pmel-1 CTLA-4–/– mice revealed that the T-cell compartment was composed of primarily CD4+ T cells (77% CD4+ T cells vs 23% CD8+ T cells; Figure 4A). Furthermore in these mice, there was a pronounced diversity of the TCR repertoire of CD4+ T cells. Most (86%) of the CD4+ T cells from the pmel-1 CTLA-4+/+ mouse expressed the transgenic Vβ13 chain, whereas most (78%) of the CD4+ T cells from the pmel-1 CTLA-4–/– mouse expressed endogenous non-Vβ13 chains (Figure 4B). Because the CD4+ T-cell population was found to be preferentially dysregulated, we sought to determine the effect of removal of the CD4+ T-cell population from pmel-1 CTLA-4–/– mice by crossing pmel-1 CTLA-4–/– mice onto a Rag-1–/– background (Figure 5A). Strikingly, the resultant offspring failed to develop vitiligo and retained a normal lifespan (Figure 5B). At 4 to 6 weeks of age, 190 pups were examined in a blinded fashion for the development of coat depigmentation. A number of mice (26) developed vitiligo over 75% of their body surface (Table 2). Again, genotypic analysis confirmed that all 26 (100%) of these mice were pmel-1 CTLA-4–/– and homoor heterozygous for the expression of the Rag1 gene. Conversely, autoimmune vitiligo was not seen in any of the 28 pmel-1+ Rag–/– CTLA-4–/– mice (P < .001; Table 2). We next analyzed the expression of activation markers on the cell surface of splenic CD8+ T cells from these mice. Again, we observed an activated phenotype in the CD8+ T-cell population of pmel-1 CTLA-4–/– mice. In contrast, CD8+ T cells from pmel-1 Rag–/– CTLA-4–/– mice showed a completely naive phenotype, with virtually undetectable levels of CD44 expression (Figure 5C). There was no functional defect in the pmel-1 CD8+ T cells when crossed onto a Rag-1–/– background, as they were equally able to induce tumor regression upon adoptive transfer (data not shown). Therefore, the enhanced self-reactivity, as seen by the rapid development of autoimmune vitiligo in pmel-1 CTLA-4–/– mice, was secondary to a CD4+ T-cell–mediated activation of the class I–restricted CD8+ T cells.
Development of autoimmune vitiligo in pmel-1 CTLA-4–/– mice is a result of qualitative rather than quantitative improvements in pmel-1 CD8+ T cells. (A) Quantitative analysis of CD8+ splenocytes in pmel-1 CTLA+/+ and pmel-1 CTLA-4–/– mice. Pmel-1 CTLA-4–/– mice have significantly fewer CD8+ T cells compared with pmel-1 mice (P < .001). (B) Pmel-1 CTLA-4–/– CD8+ T cells exhibit an activated phenotype. Flow cytometry for CD44 expression on CD8+ splenocytes from pmel-1 CTLA+/+ and pmel-1 CTLA-4–/– mice. Results are shown after gating for CD8. (C) Pmel-1 CTLA-4–/– CD8+ T cells possess enhanced effector functions. Splenocytes from pmel-1 CTLA+/+ and pmel-1 CTLA-4–/– mice were enriched for CD8+ T cells and cocultured in the presence of gp100-pulsed, irradiated, T-cell–depleted splenocytes. Irradiated, unpulsed, T-cell–depleted splenocytes were used as controls. Pmel-1 CTLA-4–/– CD8+ T cells release significantly greater amounts of IFN-γ (P < .001). (A, C) Values represent mean ± SEM.
Development of autoimmune vitiligo in pmel-1 CTLA-4–/– mice is a result of qualitative rather than quantitative improvements in pmel-1 CD8+ T cells. (A) Quantitative analysis of CD8+ splenocytes in pmel-1 CTLA+/+ and pmel-1 CTLA-4–/– mice. Pmel-1 CTLA-4–/– mice have significantly fewer CD8+ T cells compared with pmel-1 mice (P < .001). (B) Pmel-1 CTLA-4–/– CD8+ T cells exhibit an activated phenotype. Flow cytometry for CD44 expression on CD8+ splenocytes from pmel-1 CTLA+/+ and pmel-1 CTLA-4–/– mice. Results are shown after gating for CD8. (C) Pmel-1 CTLA-4–/– CD8+ T cells possess enhanced effector functions. Splenocytes from pmel-1 CTLA+/+ and pmel-1 CTLA-4–/– mice were enriched for CD8+ T cells and cocultured in the presence of gp100-pulsed, irradiated, T-cell–depleted splenocytes. Irradiated, unpulsed, T-cell–depleted splenocytes were used as controls. Pmel-1 CTLA-4–/– CD8+ T cells release significantly greater amounts of IFN-γ (P < .001). (A, C) Values represent mean ± SEM.
Lack of CTLA-4 expression does not enhance antitumor efficacy of adoptively transferred self/tumor-reactive CD8+T cells. (A) C57BL/6 mice bearing 10-day-old established subcutaneous B16 tumors were left untreated as control or received adoptive transfer of 1 × 106 cultured pmel-1 or pmel-1 CTLA-4–/– T cells in conjunction with rFPhgp100 vaccination and exogenous rhIL-2 (36 μg per dose). (B) Sublethally irradiated mice (5 Gy) received 1 × 106 cultured pmel-1 CTLA+/+ or pmel-1 CTLA-4–/– T cells. The following day, mice were challenged with 5 × 105 B16 cells. Tumor area results are the mean of measurements from 5 mice per group (± SEM).
Lack of CTLA-4 expression does not enhance antitumor efficacy of adoptively transferred self/tumor-reactive CD8+T cells. (A) C57BL/6 mice bearing 10-day-old established subcutaneous B16 tumors were left untreated as control or received adoptive transfer of 1 × 106 cultured pmel-1 or pmel-1 CTLA-4–/– T cells in conjunction with rFPhgp100 vaccination and exogenous rhIL-2 (36 μg per dose). (B) Sublethally irradiated mice (5 Gy) received 1 × 106 cultured pmel-1 CTLA+/+ or pmel-1 CTLA-4–/– T cells. The following day, mice were challenged with 5 × 105 B16 cells. Tumor area results are the mean of measurements from 5 mice per group (± SEM).
Lack of CTLA-4 expression in pmel-1 CTLA-4–/– mice results in dysregulation of CD4+ T cells. (A) CD4+ T-cell population is overrepresented in pmel-1 CTLA-4–/– mice. Flow cytometry for CD4 and CD8 expression on splenocytes from pmel-1 CTLA+/+ and pmel-1 CTLA-4–/– mice. Results are shown after lymphocyte gating and are representative of 2 independent experiments. (B) Lack of CTLA-4 expression in pmel-1 CTLA-4–/– results in dysregulation of the CD4+ TCR repertoire. Flow cytometry for CD4 and Vβ13 expression on splenocytes from pmel-1 CTLA+/+ and pmel-1 CTLA-4–/– mice. Results are shown after lymphocyte gating.
Lack of CTLA-4 expression in pmel-1 CTLA-4–/– mice results in dysregulation of CD4+ T cells. (A) CD4+ T-cell population is overrepresented in pmel-1 CTLA-4–/– mice. Flow cytometry for CD4 and CD8 expression on splenocytes from pmel-1 CTLA+/+ and pmel-1 CTLA-4–/– mice. Results are shown after lymphocyte gating and are representative of 2 independent experiments. (B) Lack of CTLA-4 expression in pmel-1 CTLA-4–/– results in dysregulation of the CD4+ TCR repertoire. Flow cytometry for CD4 and Vβ13 expression on splenocytes from pmel-1 CTLA+/+ and pmel-1 CTLA-4–/– mice. Results are shown after lymphocyte gating.
CD8+CLTA-4–/–T-cell–mediated autoimmunity required CD4+CLTA- 4–/–T cells. (A) Lack of CD4+ T cells in pmel-1 Rag-1–/– CTLA-4–/– mice. Flow cytometry for CD4 and CD8 expression on splenocytes from pmel-1 Rag-1–/– CTLA-4–/– mice. Results are shown after lymphocyte gating. (B) Absence of coat depigmentation in pmel-1 Rag–/– CTLA-4–/– mice. Pmel-1 Rag–/– CTLA-4–/– (left) and pmel-1 CTLA-4–/– (right) littermates are depicted at 10 weeks of age. Autoimmune vitiligo is present over more than 75% of the body surface of pmel-1 CTLA-4–/– mice. (C) Pmel-1 Rag–/– CTLA-4–/– CD8+ T cells exhibit a naive phenotype. Flow cytometry for CD44 expression on CD8+ splenocytes from pmel-1 CTLA-4–/– and pmel-1 Rag–/– CTLA-4–/– mice. Results are shown after gating for CD8.
CD8+CLTA-4–/–T-cell–mediated autoimmunity required CD4+CLTA- 4–/–T cells. (A) Lack of CD4+ T cells in pmel-1 Rag-1–/– CTLA-4–/– mice. Flow cytometry for CD4 and CD8 expression on splenocytes from pmel-1 Rag-1–/– CTLA-4–/– mice. Results are shown after lymphocyte gating. (B) Absence of coat depigmentation in pmel-1 Rag–/– CTLA-4–/– mice. Pmel-1 Rag–/– CTLA-4–/– (left) and pmel-1 CTLA-4–/– (right) littermates are depicted at 10 weeks of age. Autoimmune vitiligo is present over more than 75% of the body surface of pmel-1 CTLA-4–/– mice. (C) Pmel-1 Rag–/– CTLA-4–/– CD8+ T cells exhibit a naive phenotype. Flow cytometry for CD44 expression on CD8+ splenocytes from pmel-1 CTLA-4–/– and pmel-1 Rag–/– CTLA-4–/– mice. Results are shown after gating for CD8.
It remained unclear whether the enhanced induction of autoimmune vitiligo that was dependent on dysregulated CD4+ T cells required the absence of CTLA-4 on CD8+ pmel-1 T cells. Purified CD4+ CTLA-4–/– T cells were cotransferred with a sorted population of pmel-1 CTLA+/+ CD8+ T cells into Rag-1–/– mice. Vitiligo was blindly evaluated after 2 months. There were no statistically significant differences in vitiligo between controls and mice that received CD4+ CTLA-4–/– T cells together with pmel-1 CTLA+/+ CD8+ T cells (Table 3). Similar results were observed in bone marrow chimeras consisting of pmel-1 CTLA-4+/+ and CTLA-4–/– (Table 4). These findings together indicated that CTLA-4 dysregulation of self/tumor-reactive CD8+ T-cell function is CD4+ T-cell dependent.
Discussion
CTLA-4 blockade provides a promising means of reducing peripheral tolerance to self-antigens and enhancing antitumor immunity. Administration of anti–CTLA-4–blocking antibodies in both murine models and clinical trials induced reductions in tumor burden and increases in the incidence of autoimmune manifestations.11-14,16 However, the cellular mechanisms underlying the blockade of CTLA-4–negative regulation remain poorly defined. Some groups have shown that CTLA-4 blockade directly enhances CD8+ T-cell activation, proliferation, and cytotoxic activity, even in the absence of CD4+ T-cell help.22,34,36-39 In contrast, we have found that the lack of CTLA-4 expression on CD8+ T cells alone is insufficient to break peripheral tolerance and enhance effector functions of self/tumor-reactive T cells. CTLA-4 blockade together with granulocyte-macrophage colony-stimulating factor (GM-CSF)–producing vaccines have been shown to induce the rejection of B16 challenge or early established tumors while it was ineffective in treating large, vascularized subcutaneous tumors such as those used in our model.34,35 However, even when tested in a preventative setting, pmel-1 CTLA-4–/– CD8+ T cells did not exhibit increased antitumor efficacy compared with pmel-1 CTLA-4+/+ CD8+ T cells. Unlike previous reports that indicate the absence of CTLA-4 had a greater impact on activated T cells rather than naive T cells we did not find that CTLA-4 blockade enhanced the function of activated pmel-1 T cells.37,40 In addition, our findings do not appear to be restricted to self-antigen systems because similar results can be found in the literature. The lack of CTLA-4 did not affect the ability of adoptively transferred 2C TCR Rag-2–/– CD8+ T cells to control the growth of B16 tumor expressing the Kb-binding peptide SIYRYGL growth.41 Moreover, in the lymphocytic choriomeningitis viral model, the absence of CTLA-4 on p33-specific transgenic TCR CD8+ T cells did not affect antiviral T-cell responses.42 Because we have found that pmel-1 CTLA-4–/– CD8+ T cells are phenotypically and functionally more differentiated than pmel-1 CTLA-4+/+ CD8+ T cells, we cannot exclude that their inability to induce greater tumor regression after adoptive transfer was due to their progressed differentiation state.31 The abrogation of autoimmune manifestations in pmel-1 Rag–/– CTLA-4–/– mice further suggests that the mere absence of CTLA-4 on CD8+ T cells alone does not trigger their activation. A similar phenomenon has been reported for a model using class II–restricted pigeon cytochrome c–specific TCR transgenic mice, called AND, that were crossed onto a CTLA-4–/– background. Expression of the TCR AND transgene by CD4+ T cells delayed but did not prevent lymphoproliferative disease in the CTLA-4–/– mice.43 Mice remained healthy and CD4+ T cells maintained a naive phenotype when AND CTLA-4–/– transgenic mice were crossed onto Rag-1–/– mice. At first glance, results in the pmel-1 model seem to recapitulate those found in the AND system, but the implications of these apparently similar findings are profoundly different. In the AND model, the lymphoproliferative autoimmune disease is interpreted to be abrogated by the elimination of self-reactive T cells deriving from the creation of monoclonal T cells specific for a foreign antigen. By sharp contrast, the robust autoimmunity manifested by vitiligo is also completely eliminated in pmel-1 mice that are entirely targeted to a self-antigen. This indicated that the lack of autoimmunity is not due to the elimination of self-reactive T cells. It would be interesting to assess the influence of CTLA-4 on the function of self-antigen–reactive, MHC class II–restricted, CD4+ TCR transgenic mice. However, we cannot exclude the possibility that the absence of T-cell activation and autoimmunity in both the pmel-1 and AND TCR models is derived from disrupted T-cell development or changes in host factors. For example, the lack of B cells in Rag-1–/– mice alters the architecture of lymphoid organs.
Consistent with Chambers et al,8 our data indicate a CD4+ T-cell–dependent mechanism of CTLA-4 immunoregulation. It appears that CD4+ T cells directly mediate autoimmune manifestations in CTLA-4–/– mice in other systems—depletion of the CD8+ T-cell population does not modify the phenotype of these mice. Conversely, in our model the CD4+ T cells are not the final mediators of autoimmunity; in the absence of pmel-1 CD8+ T cells, CTLA-4–/– mice do not develop vitiligo. Similar to our findings, Shrikant et al13 have reported that CTLA-4 blockade reverses CD8+ T-cell tolerance to tumor through a CD4+ T-cell–dependent mechanism. In addition, analyses on peripheral blood lymphocytes of patients treated with anti–CTLA-4 have revealed a stronger up-regulation of the activation marker human leukocyte antigen (HLA)–DR and memory marker CD45RO+ on CD4+ T cells compared with CD4– T cells, suggesting a preferential activation of the CD4+ T-cell population following CTLA-4 blockade.16,17
Dysregulated CD4+ CTLA-4–/– T cells were unable to activate and trigger pmel-1 CD8+ CTLA+/+ T cells to mediate autoimmunity upon cotransfer into Rag-1–/– mice or in BM mixed chimeras. The inability of CD4+ CTLA-4–/– T cells to recapitulate the phenotype of pmel-1 CTLA-4–/– mice in the presence of CTLA-4 WT pmel-1 CD8+ T cells suggests that the absence of CTLA-4 on CD8+ T cells is required. However, we cannot resolve alternative possibilities, which include: (1) the lack of CTLA-4 lowers the threshold of CD4+ T-cell–dependent CD8+ T-cell activation; (2) the presence of CTLA-4 on CD8+ T cells inhibits the dysregulation of the CD4+ CTLA-4–/– T cells by providing CTLA-4 inhibition in trans44 ; or (3) in the absence of CTLA-4, CD8+ T cells fail to release unidentified suppressive factors. These alternative explanations apply to experiments in which CTLA-4–/–, CTLA-4+/+ BM mixed chimeras exhibit a normal phenotype42 and no augmentation in CD8+ or CD4+ T-cell–mediated antiviral responses.45
In summary, our results indicated that CD4+ T cells, whose function is dysregulated by the absence of CTLA-4–mediated negative costimulation, trigger the induction of CD8+ T-cell–mediated autoimmunity and tumor immunity, but only if these CD8+ T cells lack CLTA-4. Thus, the successful treatment of patients with antibodies that block CTLA-4 may be the result of the dysregulation of self/tumor-reactive CD8+ and CD4+ T cells acting in concert.
Authorship
The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 1, 2006; DOI 10.1182/blood-2006-07-034066.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
The authors would like to thank James P. Allison for providing the CTLA-4–/– mice and for critical review of the manuscript. This work was performed in partial fulfillment of a PhD (D.C.P.) at George Washington University, Washington, DC.
This work was supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.