Abstract
The mammalian target of rapamycin (mTOR) is emerging as a promising target for antitumor therapy. However, the mechanism that contributes to its regulation in B lymphomas remains unknown. This study shows that in follicular lymphoma (FL) cells, mTOR is active because the cells displayed rapamycin-sensitive phosphorylation of p70S6 kinase and 4E-BP1. Moreover, immunohistochemistry applied on lymph node tissue sections obtained from patients with FL revealed that, in most cases, p70S6 kinase was highly phosphorylated compared to normal tonsillar tissue. In FL cells, mTOR was under control of both phospholipase D (PLD) and phosphatidylinositol 3-kinase (PI3K). Moreover, we demonstrated that Syk plays a central role in mTOR activation because we found that both expression and activity are elevated compared to normal or chronic lymphocytic leukemia B cells. We also provide evidence that Syk operates through PLD- and PI3K-independent pathways. Finally, Syk inhibition by piceatannol or by siRNA plasmids resulted in a potent inhibition of mTOR activity in FL cells, as well as in mantle cell lymphoma, Burkitt lymphoma, and diffuse large B-cell lymphoma. These findings suggest that the Syk-mTOR pathway has a critical function in FL survival, and therefore, that Syk could be a promising new target for B-lymphoma therapy.
Introduction
Non-Hodgkin lymphoma (NHL) is the most commonly occurring malignant blood disease. Eighty-five percent of NHL belongs to the B lineage, and the most commonly occurring variety includes the diffuse large B-cell lymphoma (DLBCL). Follicular lymphoma (FL) is the second most common type of B-NHL and includes 35% to 40% of all adult lymphomas. FL is derived from germinal center follicle lymphoid cells.1 FL is characterized by an indolent initial course, a favorable response to first-line therapy, and also recurrences followed by a refractory phase eventually complicated by histologic transformation. Although the prognosis of FL is variable, most patients with aggressive forms of FL (high FLIPI score) ultimately die from their disease and median survival is 5 to 8 years.2 Despite the significant progress that has been made, mostly due to the introduction of rituximab combined with chemotherapy, further efforts are needed to identify new molecular targets in FL.
In the vast majority of cases, FL cells display high expression of Bcl-2 as a consequence of t(14;18).3,4 Based on the potent antiapoptotic properties of this protein, it has been proposed that not only is Bcl-2 a major, if not unique, causal factor for FL, but also that Bcl-2 overexpression represents a major obstacle for the eradication of tumor cells by chemotherapy, including that used in preparative regimens before stem cell transplantation.5 However, approximately 10% to 15% of patients with FL are negative for Bcl-2.6 Interestingly, clinical presentation, histopathologic findings, response to therapy, and clinical outcome in Bcl-2+ patients are indistinguishable from those in Bcl-2– FL patients. This observation suggests that, independently from Bcl-2, FL cells display alternative antiapoptotic pathways that ultimately interfere with the response to therapy. The recent identification of constitutively phosphorylated forms of AKT in FL histologic specimens supports this hypothesis.7
The mammalian target of rapamycin, mTOR, is a 289-kDa evolutionarily conserved serine/threonine kinase that interacts and phosphorylates the ribosomal protein S6 kinase (p70S6K1) and the eukaryotic initiation factor 4E-binding protein (4E-BP), 2 proteins involved in the regulation of protein synthesis.8,9 mTOR is under control of at least 2 main signaling pathways, including the PI3K/AKT pathway and the classical MAPK/ERK module.10,11 mTOR activation results in the phosphorylation of p70S6K on a specific site: Thr389. In addition, other studies have demonstrated that phopholipase D (PLD) is implicated in mTOR-dependent survival signals12-14 through the interaction of phosphatidic acid with the FKBP12-rapamycin–binding domain of mTOR.15
Although its regulation is not totally elucidated, mTOR has emerged as a major player in many aspects of cell biology, including membrane trafficking and protein degradation, but more importantly, cell cycle progression and survival.8,9 For this reason, a number of mTOR inhibitors, including rapamycin or rapamycin analogues have been introduced in clinical oncology with some encouraging results,16-18 including in acute myeloid leukemia,19 mantle cell lymphoma (MCL),20,21 and Burkitt lymphoma.22 However, these drugs had received little if any attention in FL cells. Moreover, to the best of our knowledge, the status of mTOR in FL cells has not yet been described. This is surprising because mTOR is a plausible candidate to regulate FL cell survival. The fact that, on one hand, the PI3K/AKT pathway is an important regulator of mTOR, and, on the other hand, AKT is activated in FL cells,7 supports this hypothesis.
Protein tyrosine kinases (PTKs) are important components of intracellular signal transduction pathways often involved in tumorigenesis of B cells.23 The predominant tyrosine kinases expressed in B-cell lines are Lyn and Syk. The latter has been implicated in the signal transduction pathways of several cell surface receptors such as the B-cell receptor (BCR).24-30 Thus, BCR activation results in rapid tyrosine phosphorylation of Syk, and this event is critical for the activation of the PI3K/AKT pathway and survival of activated B cells.31 The influence of Syk on the PI3K/AKT pathway renders possible its contribution to mTOR regulation. Moreover, independent studies have shown that, in B cells, BCR-mediated Syk activation results in the stimulation of PLD activity, another contributor of mTOR regulation.32,33
Based on these findings and considerations, we hypothesized that Syk function is deregulated in FL cells and also that Syk activation results in the stimulation of the mTOR pathway, possibly through PI3K/AKT or PLD pathways.
Materials and methods
Cell lines and reagents
RL and Karpas-422 are FL cells carrying t(14;18). Raji is a Burkitt lymphoma cell line carrying t(8;14). RL and Raji were obtained from the American Type Cell Collection (ATCC; Rockville, MD) and Karpas-422 from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ) cell collection (Braunschweig, Germany). Granta 519 and Rec-1 are MCL cells carrying t(11;14) and were a gift of Dr Pelletier and Dr Hermine (Necker, Paris, France). These cell lines, except Granta 519, were cultured at 37°C in 5% CO2 in RPMI supplemented with 10% FCS, glutamine (2 mM), streptomycin (10 μg/mL), and penicillin (200 U/mL; Invitrogen, Cergy Pontoise, France). For RL culture, HEPES (5 mM) and sodium pyruvate (25 mM; Invitrogen) were added. DEAU and LIB were derived from DLBCL34 and were kindly provided by Dr Al Saati and Pr Delsol (INSERM U563, Toulouse, France). These cell lines and Granta 519 were cultured at 37°C in 5% CO2 in IMDM supplemented with 20% FCS, glutamine (2 mM), streptomycin (10 μg/mL), and penicillin (200 U/mL).
Chronic lymphocytic leukemia (CLL) B cells were collected from peripheral blood of patients with B-CLL after informed consent was obtained (service d'hématologie, CHU Purpan, Toulouse, France); cells were separated by Ficoll-Hypaque density gradient. For each sample, flow cytometry analysis revealed that CLL cells displayed a common CD19+CD5+ B-CLL phenotype.
Human cord blood CD19+ B cells isolated from different healthy donors were obtained from Cambrex (Verviers, Belgium). These B lymphocytes are used as normal B cells in our experiments and are designed as BL.
Rapamycin, piceatannol, herbimycin A, SU6656, wortmannin, LY294002, and butanol (primary and tertiary) were purchased from Sigma Aldrich (St Quentin Fallavier, France). RAD001 was kindly provided by Novartis (Basel, Switzerland). Exponential growing cells were treated with these reagents in culture medium. DMSO was used as negative control for piceatannol or RAD001 treatment.
Syk siRNA and scramble transfection
RL cells were stably transfected in serum-free medium by electroporation at 0.25 kV and 960 μF with 20 μg Syk siRNA or scramble plasmid (Imgenex, San Diego, CA). Cells were then cultured in complete medium, and after 5 days, positive cells were selected with 0.5 mg/mL G418 (Invitrogen) during 7 days. Then, cloning was performed by plating cells in 96 wells at 0.3 cell/well. We obtained 3 clones named RL-6G2, RL-6F2, and RL-6G8 for Syk siRNA and one clone named RL-12a for scramble. Inhibition of Syk expression in RL-6G2, RL-6F2, and RL-6G8 compared to RL-12a was visualized by reverse transcription–polymerase chain reaction (RT-PCR) and Western blotting.
RT-PCR
Total cellular RNA of clones was extracted with TRIzol. RT-PCR was performed according to the manufacturer's protocol with the SuperScript one-step RT-PCR system (Invitrogen). Briefly, PCRs were run with the following cycling parameters: 50°C for 30 minutes, 95°C for 5 seconds, 32 cycles of 95°C for 1 minute, 58°C for 45 seconds, and 72°C for 1 minute. A final extension was performed at 72°C for 5 minutes. A 625-bp PCR product was obtained with Syk primer sequences following (forward 5′-TCAGCGGGTGGAATAATCTC-3′, reverse 5′-TGTGCACAAAATTGCTCTCC-3′).
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue sections from reactive or FL-affected lymph nodes were mounted on Superfrost-plus slides. Prior to immunodetection, specimens were deparaffinized and rehydrated. Endogenous peroxidase and biotin activities were blocked with specific reagents. Antigen retrieval of dewaxed sections was performed by heat treatment in 10 mM citrate buffer at pH 6. Sections were incubated for 30 minutes at room temperature with a polyclonal antiphospho-p70S6K (Thr389; Cell Signaling Technology, St Quentin Yvelines, France) diluted at 1:50 in PBS containing 0.3% BSA. Antibody binding was revealed by the streptavidinbiotin-peroxidase complex method using the Strepta ABComplex/HRP duet kit (DakoCytomation, Trappes, France). Sections were counterstained with hematoxylin and mounted with mounting media. Phospho-p70S6K was detected with a Leica DMR microscope (Rueil-Malmaison, France and IM50 software (Leica).
Western blotting
Cells (1 × 106) were washed in PBS before addition of Laemmli sample buffer (2% SDS, 10% glycerol, 5% β-mercaptoethanol, 60 mM Tris, pH 6.8, 0.001% bromophenol blue). Samples were sonicated for 15 to 20 seconds and boiled 5 minutes at 95°C. Proteins were separated on 10% or 15% SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes (Hybond C-extra, Amersham Biosciences, Cergy Pontoise, France). Nonspecific-binding sites were blocked in PBS 0.1% Tween-20 and 3% BSA. Membranes were then incubated with primary antibody overnight at 4°C, and antigen-antibody complexes were detected by enhanced chemiluminescence (ECL) system kit (Amersham Pharmacia Biotech, Saclay, France). All experiments were carried out independently at least 3 times. The different antibodies used in this study were anti-p70S6K, antiphospho-p70S6K (Thr389), anti–4E-BP1, antiphospho-4E-BP1 (Thr70), anti-AKT, antiphospho-AKT (Thr308), and anti-Syk, antiphospho-Syk (Tyr525/526), all from Cell Signaling Technology. Horseradish peroxidase–conjugated secondary antibodies against rabbit immunoglobulins were from Jackson ImmunoResearch Laboratories (Baltimore, PA).
Immunoprecipitation
Cells (10 × 106) were pelleted and then lysed with 500 μL lysis buffer (50 mM HEPES, pH 7, 150 mM NaCl, 1% Triton X-100, 10% glycerol, 1.5 mM MgCl2, 10 mM Na4P2O7, 1 mM EGTA, 10 mM DTT, 1 mM NaVO3, 1 mM PMSF, 100 mM NaF, 10 μg/mL leupeptin, and 10 μg/mL aprotinin). Protein extracts (500 μg) were incubated with 3 μg antiphosphotyrosine PY100 (Cell Signaling Technology) antibody overnight at 4°C. Immune complexes were collected by incubation with 20 μg protein G Sepharose beads (Amersham Biosciences) for 2 hours at 4°C and eluted by boiling for 5 minutes in Laemmli buffer. Western blotting anti-Syk was then performed on immunoextracts as described previously (see “Western blotting”).
Syk kinase assay
After Syk immunoprecipitation, the beads were collected by centrifugation and washed twice in wash buffer A (25 mM HEPES, pH 7.5, 150 mM NaCl, 0.1% Triton X100, 1 mM Na3VO4, 10 μg/mL aprotinin, and 10 μg/mL leupeptin) and twice in wash buffer B (25 mM HEPES, pH 7.5, 150 mM NaCl, 1 mM Na3VO4, 10 μg/mL aprotinin, and 10 μg/mL leupeptin). The beads were incubated with 40 μL kinase mix containing 25 mM HEPES, pH 7.5, 2 mM MnCl2, 20 mM P-nitrophenyl phosphate, 10 μM ATP, and 25 μCi (0.925 MBq) γ-32P]ATP for 10 minutes at 30°C. The reaction was stopped by the addition of 15 μL 4X Laemmli sample buffer, and then samples were boiled for 5 minutes. Proteins were separated on 10% SDS-PAGE gels and transferred to nitrocellulose membranes. Radioactive bands corresponding to Syk autophosphorylation were visualized by a Phosphorimager (Molecular Dynamics, Piscataway, NJ).
PI3K kinase assay
PI3K activity was measured in a final volume of 100 μL containing 50 mM Tris-HCl, pH 7.4, 1.5 mM DTT, 100 mM NaCl, 0.5 mM EDTA, 5 mM MgCl2, 100 μM ATP, 25 μCi (0.925 MBq) γ-32P]ATP, exogenous lipid vesicles (100 μM phosphatidylinositol 4,5-bisphosphate plus 200 μM phosphatidylserine, prepared by sonication in 50 mM Tris-HCl, pH 7.4), and p85 immunoprecipitates. After 10 minutes at 37°C, reactions were stopped by adding HCl 1 N, then a mixture of chloroform/methanol (vol/vol). Lipids were extracted as described35 and separated by thin-layer chromatography (Merck, West Point, PA) using chloroform/acetone/methanol/acetic acid/water (80:30:26:24:14 vol/vol) and the radioactive spots corresponding to 32P-PIP3 were visualized by a Phosphorimager (Molecular Dynamics).
PLD kinase assay
Cells (1 × 106) were suspended in 250 μL ice-cold PBS containing Ca2+ and Mg2+ and supplemented with 2.5 μL 1 M HEPES solution, 1.17 nmol Bodipy-PC (Molecular Probes, Leiden, The Netherlands) and 2% vol/vol ethanol. After 1 hour of incubation at 37°C with gentle mixing, total lipids were extracted with 1 mL ice-cold butanol. Samples were then evaporated to dryness and the residue was dissolved in 150 μL of a hexane/isopropanol/acetic acid/triethylamine mixture (50:50:1:0.08 vol/vol). High-performance liquid chromatography (HPLC) separation and quantification of Bodipy-PC breakdown products was performed according to a slightly modified method of Kemken et al.36
Clonogenic assay
Cells (5 × 103) were incubated with increasing doses of rapamycin or piceatannol in 35-mm Petri dishes and grown in H4230 Stem Cell Technologies (Vancouver, BC, Canada) methyl cellulose medium in a humidified CO2 incubator (37°C). After 7 days, B-lymphoma colonies (> 20 cells) were scored under an inverted microscope. Clonogenic efficiency was measured by dividing clone number per number of cells plated.
Results
mTOR is active in FL cells
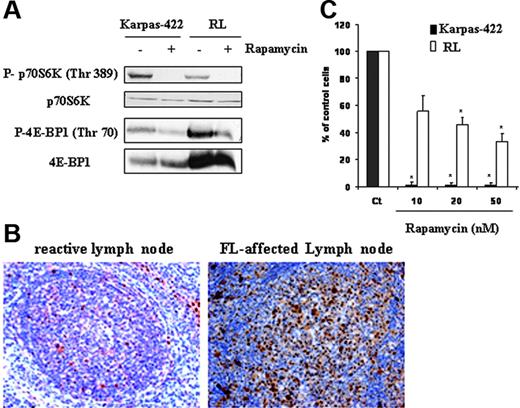
We evaluated mTOR activity in Karpas-422 and RL cell lines by measuring the phosphorylation profile of its substrates p70S6K and 4E-BP1 by Western blot analysis using antibodies directed against mTOR-specific phosphorylation sites for each protein, respectively, Thr389 and Thr70. As shown in Figure 1A, we found that FL cells displayed a high expression of phosphorylated forms of p70S6K and 4E-BP1 as well as mTOR on Ser2481 (data not shown). As expected, treatment with rapamycin (10 nM for 24 hours) resulted in the abrogation of both p70S6K and 4E-BP1 phosphorylation in Karpas-422 and RL cells (Figure 1A). Similar results were obtained with a clinical analogue of rapamycin, RAD001 (data not shown). For the rest of our experiments, we examined mTOR activity by measuring the phosphorylation of p70S6K. To evaluate the relevance of these observations in patients with FL, we investigated the phosphorylation status of p70S6K by immunohistochemistry applied to lymph node tissue sections. As shown in Figure 1B, in most cases (6 of 8 samples), FL cells displayed an intense nuclear and cytoplasmic staining with an antibody directed against p70S6K phosphorylated form, compared to tonsillar B cells issued from healthy donors. We further investigated the implication of the mTOR pathway on clonogenicity of FL cells. As observed after 7 days in methylcellulose cultures, treatment with rapamycin at 20 nM resulted in a significant reduction (98% and 54%) of clone formation in Karpas-422 and RL cells, respectively (Figure 1C). Clonogenicity of Karpas-422 and RL cells was also inhibited (94% and 48%, respectively) when cells were treated with RAD001 (data not shown).
mTOR activity in cells and tissue derived from FL. (A) FL cell lines (Karpas-422 and RL) were treated or not with rapamycin at 10 nM for 24 hours, then mTOR activity was evaluated by Western blotting using antiphospho-p70S6K and phospho-4E-BP1 antibodies. p70S6K and 4E-BP1 were used as controls of protein expression. Results are representative of 3 independent experiments. (B) mTOR activity was evaluated in FL lymph node sections by immunohistochemistry using an antiphospho-p70S6K antibody as described in “Materials and methods.” This result is representative of the 6 positive samples analyzed. Objective magnification 20×/0.5 NA. (C) Clonogenicity of FL cells treated or not with rapamycin at various doses was measured after 7 days in methylcellulose medium as described in “Materials and methods.” CT corresponds to untreated FL cells, and results are the mean ± SD of 3 independent experiments. * P < .01.
mTOR activity in cells and tissue derived from FL. (A) FL cell lines (Karpas-422 and RL) were treated or not with rapamycin at 10 nM for 24 hours, then mTOR activity was evaluated by Western blotting using antiphospho-p70S6K and phospho-4E-BP1 antibodies. p70S6K and 4E-BP1 were used as controls of protein expression. Results are representative of 3 independent experiments. (B) mTOR activity was evaluated in FL lymph node sections by immunohistochemistry using an antiphospho-p70S6K antibody as described in “Materials and methods.” This result is representative of the 6 positive samples analyzed. Objective magnification 20×/0.5 NA. (C) Clonogenicity of FL cells treated or not with rapamycin at various doses was measured after 7 days in methylcellulose medium as described in “Materials and methods.” CT corresponds to untreated FL cells, and results are the mean ± SD of 3 independent experiments. * P < .01.
These results show that, in FL, mTOR is activated and plays an important role for cell survival.
Syk is a regulator of mTOR in FL cells
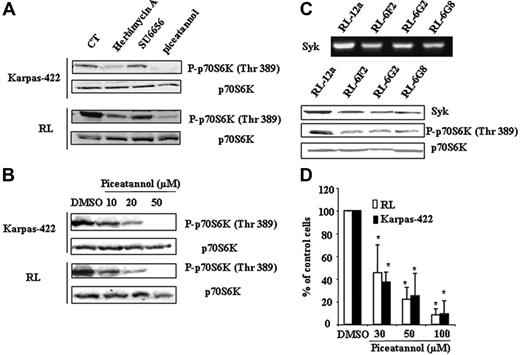
We evaluated the effect of different tyrosine kinase inhibitors, including herbimycin A (pan-tyrosine kinase inhibitor), SU6656 (Src inhibitor), and piceatannol (Syk inhibitor), on p70S6K phosphorylation. As shown in Figure 2A, SU6656 had no effect, suggesting that Scr plays a little, if any, role in mTOR regulation in FL cells. In contrast, both herbimycin A and piceatannol strongly inhibited p70S6K phosphorylation. The inhibitory effect of piceatannol on p70S6K phosphorylation was dose-dependent (Figure 2B). To confirm the role of Syk in mTOR regulation, we investigated the effect of Syk depletion on p70S6K phosphorylation in FL cells using siRNA. Among the selected clones, the magnitude of Syk depletion was variable and 3 clones were selected based on the minimal Syk RNA and protein expression level. As shown in Figure 2C, Syk depletion resulted in a significant reduction of p70S6K phosphorylation. Moreover, we observed that piceatannol induced a dose-dependent reduction of FL cell clonogenicity (Figure 2D).
Implication of Syk in mTOR activity in FL cells. (A) Karpas-422 and RL cells were treated or not with herbimycin A (0.5 μg/mL), SU6656 (1 μM), or piceatannol (50 μM) for 2 hours, then p70S6K phosphorylation was evaluated by Western blotting. p70S6K was used as control of protein expression. CT corresponds to untreated FL cells and results are representative of 3 independent experiments. (B) FL cells were treated or not with piceatannol at various doses for 2 hours, and mTOR activity was then evaluated by immunoblotting using antiphospho-p70S6K antibody. p70S6K was used as control of protein expression. Results are representative of 3 independent experiments. (C) mTOR activity was evaluated on scramble (RL-12a) and Syk siRNA (RL-6G2, RL-6F2, and RL-6G8) stable transfected clones by Western blotting using antiphospho-p70S6K (Thr389) antibody. p70S6K was used as control of protein expression. Syk expression visualized by RT-PCR (left) and by Western blotting (right) was used as control for Syk depletion. (D) Clonogenicity of FL cell treated or not with piceatannol at various doses was observed after 7 days in methylcellulose medium as described in “Materials and methods.” Results are the mean ± SD of 3 independent experiments. *P < .01.
Implication of Syk in mTOR activity in FL cells. (A) Karpas-422 and RL cells were treated or not with herbimycin A (0.5 μg/mL), SU6656 (1 μM), or piceatannol (50 μM) for 2 hours, then p70S6K phosphorylation was evaluated by Western blotting. p70S6K was used as control of protein expression. CT corresponds to untreated FL cells and results are representative of 3 independent experiments. (B) FL cells were treated or not with piceatannol at various doses for 2 hours, and mTOR activity was then evaluated by immunoblotting using antiphospho-p70S6K antibody. p70S6K was used as control of protein expression. Results are representative of 3 independent experiments. (C) mTOR activity was evaluated on scramble (RL-12a) and Syk siRNA (RL-6G2, RL-6F2, and RL-6G8) stable transfected clones by Western blotting using antiphospho-p70S6K (Thr389) antibody. p70S6K was used as control of protein expression. Syk expression visualized by RT-PCR (left) and by Western blotting (right) was used as control for Syk depletion. (D) Clonogenicity of FL cell treated or not with piceatannol at various doses was observed after 7 days in methylcellulose medium as described in “Materials and methods.” Results are the mean ± SD of 3 independent experiments. *P < .01.
These results show that Syk is a critical regulator of mTOR and clonogenicity in FL cells.
Status of Syk in FL cells
Based on the preeminent role of Syk in the survival of FL cells, we investigated Syk expression level and activity in Karpas-422 and RL cells. As shown in Figure 3A, we found that Syk expression was modestly but significantly increased in FL cells, compared to normal (BL) and CLL B cells. In contrast, we found that in FL cells but not in CLL or normal B cells, Syk is mostly expressed as its active form, as attested by antiphosphotyrosine immunoprecipitation revealed by anti-Syk antibody (Figure 3B). As expected, piceatannol inhibited in a dose-dependent manner Syk phosphorylation as judged by immunoblot using an antibody directed against phospho-Syk on tyrosine 525/526 (Figure 3C) as well as by immunokinase assay (Figure 3D). It is important to note that the inhibitory effect of piceatannol on Syk phosphorylation correlates to its inhibitory effect on mTOR activity and clonogenicity (Figure 2B,D).
Status of Syk in FL cells. (A) Syk expression was visualized by Western blotting in FL cell lines and compared to B cells isolated from B-CLL patients (B-CLL) and healthy donors (BL). Actin was used as control of protein expression. Results are representative of 3 independent experiments. (B) Syk activity was evaluated by P-Tyr immunoprecipitation followed by Western blotting using an anti-Syk antibody, in FL cells and B cells isolated from healthy donors (BL) and from B-CLL patients (B-CLL). Results are representative of 3 independent experiments. (C) Effect of piceatannol on Syk activity was evaluated in Karpas-422 cells by Western blotting using an antiphospho-Syk antibody specific to tyrosine 525/526 residue. Results are representative of 3 independent experiments. (D) Effect of piceatannol on Syk activity was evaluated in RL cells by immunokinase assay as described in “Materials and methods.” Results are representative of 3 independent experiments.
Status of Syk in FL cells. (A) Syk expression was visualized by Western blotting in FL cell lines and compared to B cells isolated from B-CLL patients (B-CLL) and healthy donors (BL). Actin was used as control of protein expression. Results are representative of 3 independent experiments. (B) Syk activity was evaluated by P-Tyr immunoprecipitation followed by Western blotting using an anti-Syk antibody, in FL cells and B cells isolated from healthy donors (BL) and from B-CLL patients (B-CLL). Results are representative of 3 independent experiments. (C) Effect of piceatannol on Syk activity was evaluated in Karpas-422 cells by Western blotting using an antiphospho-Syk antibody specific to tyrosine 525/526 residue. Results are representative of 3 independent experiments. (D) Effect of piceatannol on Syk activity was evaluated in RL cells by immunokinase assay as described in “Materials and methods.” Results are representative of 3 independent experiments.
Role of Syk in PI3K/AKT-mediated mTOR regulation in FL cells
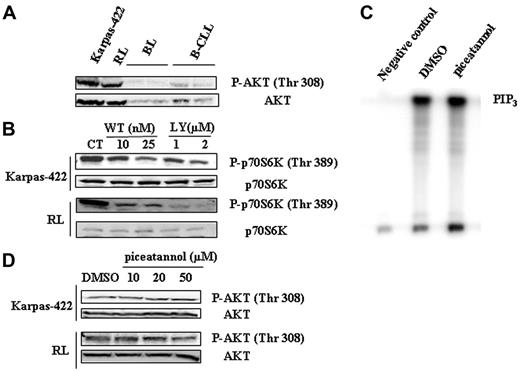
An important well-documented regulator of mTOR is the PI3K/AKT pathway (for a review, see Fingar and Blenis8 ). Moreover, AKT has been recently found to be constitutively phosphorylated in FL tissue sections.7 Based on these considerations and our results, it was therefore tempting to speculate that, in FL cells, not only did PI3K/AKT pathway play a role in mTOR activation but also that Syk acted as an upstream regulator of this pathway. We examined the status of phosphorylation of AKT on Thr308, a specific phosphorylation site for PDK1. As shown in Figure 4A, AKT is heavily phosphorylated on Thr308 compared to B-CLL and normal B cells. As expected, inhibition of PI3K by 2 inhibitors, LY294002 and wortmannin, decreased mTOR activity (Figure 4B), as well as AKT phosphorylation (data not shown) in Karpas-422 and RL cells. Wortmannin was used at low concentrations to avoid a possible inhibitory effect on mTOR as documented elsewhere.37 However, treatment with piceatannol had no impact on PI3K activity in RL cells, as evaluated by the quantification of PIP3 products by immunokinase assay (Figure 4C). Moreover, Western blotting studies revealed that Syk inhibition had no influence on AKT phosphorylation (Thr308) in Karpas-422 and RL cells (Figure 4D).
Altogether, these results suggest that, although the PI3K/AKT pathway is indeed an important regulator of mTOR in FL cells, it appears that Syk influences mTOR activity independently from this pathway.
Role of Syk in PLD-mediated mTOR regulation in FL cells
Beside PI3K, PLD is also an important mTOR regulator.13,15 Moreover, it has been established that Syk may influence PLD activity.32,33 Based on these considerations, we speculated that, in FL cells, not only did PLD play a role in mTOR activation but also that Syk acted as an upstream regulator of PLD. As shown in Figure 5A, Karpas-422 and RL cells displayed higher PLD activity than seen in normal or CLL B cells. RT-PCR analysis revealed that PLD2 was much more expressed than PLD1 (data not shown), suggesting that the former, PLD2, was the major contributor for PLD activity in FL cells. We further examined the role of PLD on mTOR activation. As shown in Figure 5B, 1-butanol, which blocks phosphatidic acid formation by PLD, inhibited the phosphorylation of p70S6K on Thr389. Moreover, 3-butanol, which does not participate in transphosphatidylation, had no impact on mTOR activity. However, treatment with piceatannol did not influence PLD activity as illustrated in Figure 5C.
Implication of PI3K/AKT pathway in mTOR activity. (A) Phosphorylation of AKT was evaluated in FL cell lines and compared to B cells isolated from healthy donors (BL) and from B-CLL patients (B-CLL) by Western blotting using anti-phospho-AKT (Thr308) antibody. AKT was used as control of protein expression. Results are representative of 3 independent experiments. (B) PI3K-dependent activation of mTOR was evaluated in Karpas-422 and RL cells treated or not with wortmannin (WT) or LY294002 for 30 minutes using antiphospho-p70S6K (Thr389) antibody. p70S6K was used as control of protein expression. CT corresponds to untreated FL cells and results are representative of 3 independent experiments. (C) The effect of Syk inhibition on PI3K activity was evaluated on RL cells. PI3K activity assay was performed as indicated in “Materials and methods” with cells treated 2 hours with 50 μM piceatannol. The PIP3 products of PI3K activation are indicated. Negative control corresponds to cell lysate incubated without p85 antibody. Results are representative of 3 independent experiments. (D) The effect of Syk inhibition on AKT phosphorylation was evaluated on Karpas-422 and RL cell lines. Cells were treated with piceatannol at various doses for 2 hours and AKT phosphorylation (Thr308) was evaluated by Western blotting. Results are representative of 3 independent experiments.
Implication of PI3K/AKT pathway in mTOR activity. (A) Phosphorylation of AKT was evaluated in FL cell lines and compared to B cells isolated from healthy donors (BL) and from B-CLL patients (B-CLL) by Western blotting using anti-phospho-AKT (Thr308) antibody. AKT was used as control of protein expression. Results are representative of 3 independent experiments. (B) PI3K-dependent activation of mTOR was evaluated in Karpas-422 and RL cells treated or not with wortmannin (WT) or LY294002 for 30 minutes using antiphospho-p70S6K (Thr389) antibody. p70S6K was used as control of protein expression. CT corresponds to untreated FL cells and results are representative of 3 independent experiments. (C) The effect of Syk inhibition on PI3K activity was evaluated on RL cells. PI3K activity assay was performed as indicated in “Materials and methods” with cells treated 2 hours with 50 μM piceatannol. The PIP3 products of PI3K activation are indicated. Negative control corresponds to cell lysate incubated without p85 antibody. Results are representative of 3 independent experiments. (D) The effect of Syk inhibition on AKT phosphorylation was evaluated on Karpas-422 and RL cell lines. Cells were treated with piceatannol at various doses for 2 hours and AKT phosphorylation (Thr308) was evaluated by Western blotting. Results are representative of 3 independent experiments.
Altogether, these results suggest that, although PLD is indeed an important regulator of mTOR in FL cells, it appears that Syk influences mTOR activity independently from this phospholipase.
Syk is responsible for mTOR activation in lymphoma cells
Finally, to investigate whether Syk-mediated mTOR activation was specific to FL or could be also observed in other malignant B-cell lymphomas, we evaluated the effect of piceatannol on p70S6K phosphorylation in different cell lines derived from MCL (Granta and Rec-1), Burkitt lymphoma (Raji), and DLBCL (DEAU and LIB). As shown in Figure 6, Syk inhibition resulted in a decrease in mTOR activity in all tested cell lines.
Implication of PLD pathway in mTOR activity. (A) PLD activity was measured in FL cell lines and compared to B cells isolated from healthy donors (BL) and from B-CLL patients (B-CLL) as described in “Materials and methods.” Results are the mean ± SD of 3 independent experiments. (B) PLD-dependent activation of mTOR was evaluated using antiphospho-p70S6K (Thr389) antibody in Karpas-422 and RL cells treated or not with 1-butanol or 3-butanol at 0.3% during a time course. p70S6K was used as control of protein expression. CT corresponds to untreated FL cells and results are representative of 3 independent experiments. (C) Syk-dependent PLD activation was evaluated by treating FL cell lines with piceatannol at 50 μM for 2 hours and measuring PLD activity as described in “Materials and methods.” Results are the mean ± SD of 3 independent experiments.
Implication of PLD pathway in mTOR activity. (A) PLD activity was measured in FL cell lines and compared to B cells isolated from healthy donors (BL) and from B-CLL patients (B-CLL) as described in “Materials and methods.” Results are the mean ± SD of 3 independent experiments. (B) PLD-dependent activation of mTOR was evaluated using antiphospho-p70S6K (Thr389) antibody in Karpas-422 and RL cells treated or not with 1-butanol or 3-butanol at 0.3% during a time course. p70S6K was used as control of protein expression. CT corresponds to untreated FL cells and results are representative of 3 independent experiments. (C) Syk-dependent PLD activation was evaluated by treating FL cell lines with piceatannol at 50 μM for 2 hours and measuring PLD activity as described in “Materials and methods.” Results are the mean ± SD of 3 independent experiments.
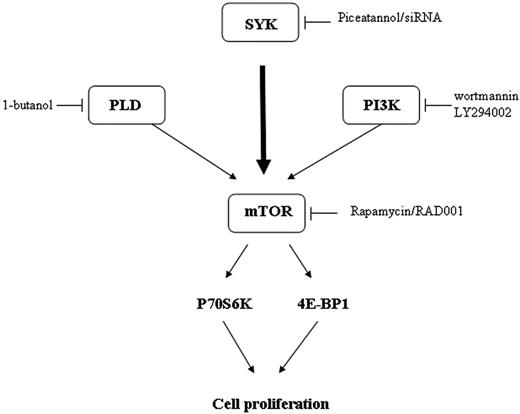
Thus, our study shows that in malignant B-cell lymphomas, Syk is a critical regulator of mTOR activity (Figure 7).
Discussion
In this study, we show for the first time that, in FL cells, as well as in other malignant B cells, mTOR is active as indicated by the phosphorylation of its substrates, p70S6K and 4E-BP1. It is, therefore, not surprising that mTOR inhibitors such as rapamycin and RAD001 displayed potent antitumor effect against FL cells. This may have important clinical implications for the rapamycin analogue now in clinical development in MCL.21 It is important to note that both Karpas-422 and RL cells are negative for EBV. Moreover, the 8 patients with FL included in our study were free from EBV. These observations suggest that, despite the fact that EBV infection may trigger the mTOR pathway,22,38 mTOR activation may also occur in EBV– malignant B cells.
The mechanism by which mTOR was activated in FL cells was also examined. As expected from previous studies based on different cellular models, we found that PI3K/AKT and PLD pathways are indeed potent regulators of mTOR in FL cells. However, as an unexpected result, we also found that Syk, a nonreceptor tyrosine kinase, is a critical component for mTOR activation operating through PI3K/AKT- and PLD-independent pathways. The role of Syk has been established using piceatannol, which is considered as specific for this kinase.39-41 Indeed, although piceatannol inhibits several other tyrosine kinases at high concentrations, at low concentrations, this drug appears to be selective for Syk, and for example, has no effect on Lyn, Hck, or Fgr activation.42 Moreover, the specificity of Syk was confirmed using siRNA stably transfected RL cells. The fact that piceatannol inhibits mTOR activity in other B-cell lymphomas, including MCL, suggests that Syk is an important regulator for mTOR signaling and cell survival in B-cell malignancies.
Dose effect of Syk inhibition on mTOR activity in malignant B-cells. Syk-dependent activation of mTOR was evaluated in MCL (Granta-519 and Rec-1), DLBCL (DEAU and LIB), and Burkitt (Raji) cell lines using antiphospho-p70S6K antibody. p70S6K was used as control of protein expression. Results are representative of 3 independent experiments.
Dose effect of Syk inhibition on mTOR activity in malignant B-cells. Syk-dependent activation of mTOR was evaluated in MCL (Granta-519 and Rec-1), DLBCL (DEAU and LIB), and Burkitt (Raji) cell lines using antiphospho-p70S6K antibody. p70S6K was used as control of protein expression. Results are representative of 3 independent experiments.
Our study shows that, in FL cells, not only is the expression of Syk higher than in normal B cells, but also that Syk is mostly expressed as its phosphorylated form, suggesting that, in lymphoma cells, Syk is dysregulated at both expression and activity levels. With regard to the Syk expression level, it is interesting to note that, using DNA or tissue microarray analysis, it has been described that, compared to normal lymphoid tissue, Syk is overrepresented in DLBCL,43 splenic marginal zone lymphoma,44 primary mediastinal B-cell lymphoma,45 and in MCL.46 In the latter study, it has been described that Syk was amplified at DNA level as validated by fluorescence in situ hybridization (FISH) analysis.46 Beside increased expression, our study suggests that Syk activity is also abnormally regulated. The mechanism that underlies the increase in Syk activity is unclear. Because, on one hand, Syk is a substrate for SHP-1 tyrosine phosphatase47 and, on the other hand, SHP-1 is often silenced in malignant B cells,48 one could speculate that SHP-1 deficiency results in elevated Syk activity. This hypothesis is currently being investigated in our laboratory. It is of interest to note that Syk status was very different in malignant B-cell lymphoma and in B-CLL. Indeed, in the latter, Syk expression is low,49 whereas Lyn, another tyrosine kinase, is overexpressed and seems to play an important role in cell survival.50 We found that Lyn expression was decreased in FL cells compared to B-CLL (data not shown), suggesting that Lyn and Syk represent 2 distinct disease-specific targets among B-cell neoplasias.
The mechanism by which Syk regulates mTOR was also examined. Despite evidence that Syk could be capable of interfering with the PLD or PI3K/AKT pathway in other cellular models, our study shows that Syk operates through another mechanism. Among other regulators, previous studies have documented that less critical, but still functional, pathways could contribute to mTOR regulation. Indeed, ERK, a component of the MAPK module, has been described as regulating mTOR activity,10 whereas other studies suggest a role for Syk in ERK regulation.42 However, we found that, although treatment with PD98059, an inhibitor of the classical MAPK module, resulted indeed in decreased p70S6K phosphorylation, piceatannol had no influence on ERK phosphorylation (data not shown). These results suggest that ERK has no role in Syk-dependent mTOR regulation. Therefore, the mechanism that links Syk and mTOR is presently unknown. A possibility is that Syk operates through TSC1/2, a proximal upstream regulator of mTOR.51,52 This hypothesis is currently being studied in our laboratory.
To conclude, our study shows that Syk contributes to mTOR activation not only in FL cells, but also in other malignant B cells, and that this pathway plays an important role in lymphoma cell survival, suggesting that Syk is a putative target for therapy in B cell-derived lymphoma.
Authorship
Contribution: L.L. performed the main body of research, analyzed the results, and wrote the paper; S.M.H. performed experiments dealing with PLD; T.alS. performed immunohistochemistry on FL specimens; F.C. performed immunohistochemistry on FL specimens; C.R. participated in experimental designs; G.L. wrote the paper; and C.B. analyzed the results and wrote the paper.
Conflict of interest disclosure: The authors declare no competing financial interests.
Prepublished online as Blood First Edition Paper, August 15, 2006; DOI 10.1182/blood-2006-05-026203.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by la Fondation de France (C.B.) and l'Association pour la Recherche sur le Cancer (G.L.). L.L. is the recipient of a grant from le Ministère déléguéà l'enseignement supérieur et à la recherche.
The authors would like to acknowledge Dr Anne Quillet-Mary for her astute advice and Dr Bernard Payrastre for critical reading of the manuscript. We also thank Dr Muriel Laffargue for help regarding PI3K activation and Drs Yves Pelletier and Olivier Hermine for providing MCL cell lines.