Abstract
Protein tyrosine phosphatase (PTPγ) is a receptor-like molecule with a known role in murine hematopoiesis. We analyzed the regulation of PTPγ expression in the human hematopoietic system, where it was detected in human peripheral blood monocytes and dendritic cells (DCs) of myeloid and plasmacytoid phenotypes. Its expression was maintained during in vitro monocyte differentiation to dendritic cells (moDC) and was further increased after maturation with bacterial lipopolysaccharide (LPS), CD40L, and TNFα. But PTPγ was absent when monocytes from the same donor were induced to differentiate in macrophages. B and T lymphocytes did not express PTPγ. Rather, PTPγ mRNA was expressed in primary and secondary lymphoid tissues, and the highest expression was in the spleen. PTPγ was detected by immunohistochemistry in subsets of myeloid-derived DCs and specialized macrophages (tingible bodies, sinus and alveolar macrophages). Classic macrophages in infective or reactive granulomatous reactions did not express PTPγ. Increased PTPγ expression was associated with a decreased ability to induce proliferation and interferon-γ secretion in T cells by moDCs from patients with advanced pancreatic cancer. Taken together, these results indicate that PTPγ is a finely regulated protein in DC and macrophage subsets in vitro and in vivo.
Introduction
Classical protein tyrosine phosphatases (PTPs) exist in transmembrane (receptor-type PTPs [RPTPs]) and nontransmembrane (non-TM) forms and have phosphotyrosine as substrate.1 RPTPs can be classified in 9 subtypes according to the different combinations of the common motifs that compose their external segments. Receptor-type protein tyrosine phosphatase gamma (PTPγ) forms with PTPζ the subtype V of RPTPs, characterized by the presence of a carbonic anhydrase-like domain and a fibronectin type III domain in the N-terminal portion of the extracellular domain.2 Hematopoietic cells express a number of RPTPs: CD45 is the prototype and is known to play a role in leukocytes, influencing the lymphocyte signaling process after antigen receptor engagement.3 Experimental evidence is now emerging concerning the involvement of a number of other RPTPs in hematopoiesis and immune response.4-8 PTPγ was previously shown to regulate hematopoietic differentiation in a murine model of hemopoietic differentiation,9 but its role in the human system is unknown.
Dendritic cells (DCs) represent a specific subset of bone marrow–derived antigen-presenting cells (APCs) that have a central role in the initiation and regulation of immune responses in lymphoid and nonlymphoid tissues.10 They are ubiquitously distributed within the body and share several common features, in particular the expression of high levels of MHC class II molecules in combination with the absence of lineage-specific markers such as CD3, CD14, CD15, CD16, CD19, CD20, and CD56 and are defined as lineage negative (lin–) cells.11 In addition to myeloid DCs (MDCs), blood DCs also include plasmacytoid DCs (PDCs). The morphology of PDCs resembles that of plasma cells. In addition, they are devoid of myeloid markers, and can be distinguished from myeloid DCs on the basis of membrane markers such as CD123, BDCA-2, and BDCA-4. After virus infection, PDCs migrate from the blood to lymph nodes, where they produce high concentrations of IFN type I.12,13 Several DC types with distinct biological features have been identified in different tissues from mice and humans, including Langerhans cells in the epidermis, interstitial DCs in various parenchyma, thymic DCs, and DC populations found in other lymphoid organs that differ in phenotype, morphology, and function.10,14-18 After activation, DCs migrate from peripheral tissues to regional lymph nodes and present the processed antigens to naive T cells.19,20 The presence of various maturation stages21,22 further complicates the picture: for example, 5 distinct DC subsets within human tonsils have been characterized.23 Like DCs, tissue macrophages are widely distributed in the human body, and they share the same microenvironmental niches. Tissue macrophages acquire markedly heterogeneous phenotypes, reflecting their specialized functions in different microenvironments.24
Here we demonstrate PTPγ expression in peripheral blood monocytes, in in vitro–differentiated DCs, and in subsets of tissue myeloid DCs and specialized macrophages. We also demonstrate that increased PTPγ expression associates with a DC phenotype characterized by a decreased capability to induce allogeneic T-cell proliferation. This represents a novel finding in the field of myeloid cell biology and might find application for the selection and identification of specific DC/specialized macrophage subsets and their activation status in vitro and in vivo with potentially relevant clinical applications.
Materials and methods
Hematopoietic cells and tissues
Circulating human monocytes (greater than 70% pure, as assessed by CD14 expression), polymorphonuclear cells (greater than 95% pure, as assessed by CD15 expression), and lymphocytes (greater than 95% pure, as assessed by morphology and the lack of CD14) were purified by Percoll (Pharmacia, Uppsala, Sweden) gradient centrifugation from leukocyte-rich buffy coats obtained from human blood of healthy donors, as described elsewhere.25 Monocytes were cultured in RPMI 1640 (2 × 106 cells/well/mL) supplemented with 2 mM glutamine and 10% heat-inactivated FCS and were maintained in a humidified atmosphere with 5% CO2 at 37°C for various times with or without indicated stimuli. Immature monocyte-derived dendritic cells (moDCs) were obtained in vitro, as previously described.26 Briefly, purified monocytes from independent donors were cultured in RPMI 1640 (2 × 106 cells/well) containing 10% heat-inactivated FCS and 2 mM glutamine and were supplemented with 50 ng/mL recombinant human GM-CSF and 20 ng/mL recombinant human IL-4 (PeproTech, Rocky Hill, NJ) for 5 to 6 days. To induce maturation, immature moDCs were treated for 24 hours with 100 ng/mL lipopolysaccharide (LPS; Escherichia coli serotype 026:B6; Sigma, St Louis, MO) or soluble CD40L (1 μg/mL) and enhancer (1 μg/mL) (Alexis Biochemical, Rome, Italy). Some of the cells were subjected to flow cytometry analysis to assess activation/maturation status.
Circulating human myeloid and plasmacytoid DCs were isolated by magnetic cell sorting with the BDCA-1 and BDCA-4 Dendritic Cell Miltenyi Biotec Isolation Kit (Miltenyi Biotec, Bologna, Italy), respectively. In all experiments, greater than 95% of the isolated cells expressed myeloid CD11c– or plasmacytoid CD123 DC–specific markers.
Mononuclear cells were isolated from nonpathologic bone marrow (provided by the Hematology Section, Verona University) by centrifugation on density gradient (Lymphoprep-Nycomed Pharma AS, Oslo, Norway).
We analyzed surgical specimens from human normal skin (obtained from plastic surgery), thymus (obtained from cardiac surgery), spleen (obtained from post-traumatic splenectomy), reactive palatine tonsils, and reactive lymph nodes (foreign body reaction to oil-based contrast media and mycobacterial lymphadenitis).
This work was conducted in accordance with a protocol approved by the Spedali Civili di Brescia, Institutional Ethical Board, and informed consent was obtained from all patients.
From each specimen, a portion was frozen in liquid nitrogen and then stored at –80°C; the remaining tissue block was formalin fixed and paraffin embedded. Cryostat sections were air dried overnight at room temperature and were fixed in acetone for 10 minutes before staining.
Twenty-four patients with inoperable or metastatic pancreatic cancer carcinoma (14 men and 10 women; median age, 68 years; range, 54-85 years) and no prior chemotherapy were enrolled in this study, which was conducted under strict observance of the principles of the Declaration of Helsinki. The diagnosis of pancreatic carcinoma was based on typical radiographic findings at ultrasonography, computed tomography, endoscopic retrograde cholangiopancreatography, or endoscopic ultrasonography. After informed consent, peripheral blood samples were obtained from patients and, for comparison, from 15 age- and sex-matched healthy donors.
RNA was extracted with TRIzol from frozen (–70°C) tissue specimens derived from a minimum of 2 spleens (trauma), thymus (cardiac surgery), tonsils (hyperplastic), lymph nodes (hyperplastic), and bone marrow (from healthy donors). RNA was treated as described for QPCR analysis.
Preparation of opsonized particles
Heat-killed Saccharomyces cerevisiae (boiled in a water bath for 30 minutes) were incubated with anti–yeast IgG, as described,27 washed twice in PBS, and stored at 4°C for 2 to 3 days.
Cytofluorometry
The following antibodies were used for the phenotyping of peripheral blood samples and DCs: mouse IgG1 anti–human CD1a, CD3, CD14, CD15, CD20, CD56, CD80, CD83, and CD86 FITC-labeled and anti-CD16, -CD11c, and -CD123 PE-labeled (BD Biosciences Pharmingen, San Diego, CA). Briefly, cells were first blocked with 10% vol/vol normal human serum (Invitrogen, Milan, Italy) for 15 minutes at room temperature and then were stained with mAb for 30 minutes at room temperature. Cells were also incubated with isotype-matched immunoglobulins (BD Biosciences PharMingen) as negative controls. Flow cytometry was performed on a Becton Dickinson FACScan flow cytometer. Analysis of flow cytometry data was performed with FCS Express V3 software (De Novo Software, Thornhill, ON, Canada).
Immunostaining of cells and tissues
Tissue blocks were cut on a cryostat into 5-μm–thick sections, mounted onto poly-l-lysine slides, air dried overnight, postfixed for 10 minutes in acetone, and stained. Primary antibodies used included PTPγ goat polyclonal IgG, C-18 (Santa Cruz Biotechnology, Santa Cruz, CA; dilution 1:20), CD3 (rabbit; Dako, Glostrup, Denmark; dilution 1:200), CD11c (LeuM5, 1:10; Becton Dickinson), CD21 (1F8, dilution 1:20; Bio-optica, Milan, Italy), CD35 (Ber-MAC-DRC, dilution 1:20; Dako), CD123 (7G3, dilution 1:40; BD Biosciences PharMingen, San Diego, CA), DC-SIGN (1:5; BD Biosciences PharMingen), DC-LAMP (104.G4, dilution, 1:100; Immunotech), and CD68 (clone PG-M1, working dilution 1:40; Dako). For immunohistochemical staining, PTPγ was incubated for 2 hours, followed by biotinylated antigoat secondary antibody (Biogenex, San Ramon, CA) and streptavidin-HRP (Biogenex). For double-immunofluorescence staining, primary antibodies were revealed using Texas red– or FITC-conjugated isotype-specific secondary antibodies (dilutions 1:75 and 1:50, respectively; Vector Laboratories, Burlingame, CA). Sections were examined with an Olympus BX60 fluorescence microscope and objectives with numeric apertures of 0.40 (10×), 0.70 (20×), 0.85 (40×), and 0.90 (60×) equipped with a DP-70 Olympus digital camera (Olympus, Melville, NY). Images were acquired using analySIS Image Processing software (Soft Imaging System, Münster, Germany). For paraffin-embedded tissue, the staining was developed with the NovoLink Polymer Detection System (Novocastra Laboratories, Newcastle, United Kingdom) according to the manufacturer's instructions.
For confocal microscopy analysis, cells were washed in PBS and centrifuged on polylysinated slides, fixed with 4% paraformaldehyde, and permeabilized in PBS containing 0.2% Tween 20. Rabbit polyclonal antibody specific for the PTPγ extracellular domain28 was used at 10 μg/mL concentration in 0.1% Tween 20-PBS and was incubated for 2 hours at room temperature. Other FITC-conjugated monoclonal antibodies (anti-CD83 [Immunotech, Marseille, France], anti–HLA DR [BD Biosciences, Milan, Italy]) were incubated for 1 hour at 4°C, together with secondary Cy3-conjugated goat antirabbit antibody (1:1000 dilution; Amersham, Milan, Italy). After final washes in PBS, preparations were mounted on the antifading 1,4-diazabicyclo[2,2,2] octane (Sigma) in PBS containing 50% glycerol. All preparations were viewed with a Zeiss LSM 510 confocal microscope equipped with argon (488 nm) and helium/neon (543 nm) excitation beams. Results were analyzed with MetaMorph software (Universal Imaging, Downingtown, PA).
Reverse transcription–polymerase chain reaction
Total RNA from 5 × 106 cells/point was prepared using TRIzol extraction kit (Invitrogen, Life Technologies, Rockville, MD) according to the manufacturer's instructions. Total RNA (1 μg) was reverse transcribed in a volume of 20 μL using oligo (dT)20 1 μM and 200 U SuperScript II (Invitrogen, Milan, Italy) at 42°C for 1 hour, as described by the manufacturer. Polymerase chain reaction (PCR) was performed in a GeneAmp PCR System 9700 (PE Applied Biosystems, Milan, Italy) for 35 cycles (30 seconds of denaturation at 94°C, 30 seconds of annealing at 60°C, and 30 seconds of elongation at 72°C) in a volume of 25 μL reaction buffer containing 0.75 U AmpliTaq (PE Applied Biosystems), 0.4 μM each primer, and 0.2 mM dNTPs (Roche, Milan, Italy). β-Actin mRNA amplification was performed for 22 cycles on the cDNA as positive control of reaction efficiency. The same procedure for RNA extraction and PCR was applied to frozen tissue samples. In this case, 10 to 20 cryostat tissue sections (20 μm each) were resuspended in TRIzol and treated as previously described. Primers used were: ACTB 170F, 5′-ATC AAG ATC ATT GCT CCT CCT G-3′; ACTB 170R, 5′-GCA ACT AAG TCA TAG TCC GCC-3′; PTPRG 958F, 5′-CGT CAC CAG TCT CCT ATT GA-3′; PTPRG 1694R, 5′-GTC TGT CAT GTC GTG GTT CC-3′; PTPRJ F, 5′-ATG CCA CCG TTT ATT CCC AAG C-3′; PTPRJ R, 5′-GAC TCG TTA TCG CTG ACT TTC C-3′; PTPRC F, 5′-CTT AGG GAC ACG GCT GAC TTC-3′; PTPRC R, 5′-GAG TGG TTG TTT CAG AGG CAT TA-3′; PTPRE F, 5′-CCG ACA GCA ACG AGA CAA CC-3′; PTPRE R, 5′-ATT CCG TTG GGC ATC TTC TTG T-3′; PTPRU F, 5′-GCT TGC TGT CCT CAT CCT TCT-3′; and PTPRU R, 5′-CAC CAT ACG CCA GAA GTC ATA G-3′.
Real-time quantitative reverse transcriptase (RT)–PCR
cDNA was analyzed for the expression of target genes by the SYBR Green I double-stranded DNA binding dye assay, using a SYBR green PCR master mix (PE Applied Biosystems) and tested in a DNA Engine Opticon 2 Continuous Fluorescence Detector (MJ Research, Ramsey, MN). Reactions were denatured for 10 minutes at 95°C and then subjected to 50 two-step amplification cycles with denaturation at 95°C for 15 seconds followed by annealing/extension at 60°C for 1 minute. Values are expressed as number of copies of specific transcript/β-actin. Cloned β-ACT and PTPγ cDNA were used as reference for cDNA quantification. Primers used were ACTB 170F; ACTB 170R (170 bp); PTPγ1F,5′-GCC TTT ACC GTC ACC CTT ATC -3′; and PTPγ1R, 5′-AAA GGT ACT ACT TAT GGG GGC -3′ (171 bp).
Data were obtained with Optical monitor version 2.02.24 (MJ Research) software and were analyzed with GraphPad Prism version 4.00 and GraphPad InStat version 3.05 (GraphPad Software, San Diego, CA).
Allogeneic T-cell proliferation assay
moDCs were used in primary MLR to stimulate allogeneic responder T cells. T cells were isolated by negative immunomagnetic depletion of nonadherent peripheral blood mononuclear cells (PBMCs) from healthy volunteers using a monoclonal antibody mixture containing anti-CD14, -CD19, -CD20, -CD16, and -CD36 (BD PharMingen) and MACS separation columns (Miltenyi Biotec; Bergisch Gladbach, Germany). Graded numbers of irradiated (30 Gy) moDC from healthy subjects or patients were mixed with allogeneic T cells (2 × 105/200 μL) in 96-well U-bottom culture plates in RPMI 1640 containing 10% heat-inactivated FCS. To determine DNA synthesis, cells were pulsed with 18.5 KBq [3H]-TdR (GBq/mmol; Du Pont-New England Nuclear, Wilmington, DE) for the last 5 hours of a 5-day culture. Cellular DNA was collected on glass fiber filters, and [3H]-TdR incorporation was measured in a β-counter. Results are expressed as mean ± SD counts per minute (cpm) in triplicate. To measure IFN-γ production, 100 μL supernatant was harvested and frozen at –70°C.
Expression of PTPγ in peripheral blood, hematopoietic tissues, and differentiated monocytes. Number of copies of PTPRG mRNA per 106 copies of ACTB estimated by QPCR analysis in peripheral blood cells of individual donors (monocytes, n = 8; PBL, n = 6; PMN, n = 5; MDCs, n = 4; plasmacytoid DCs, n = 4) (A) and in primary and secondary hematopoietic tissue (spleen, n = 3 subjects; thymus, n = 2 subjects; tonsil, n = 2 subjects; lymph nodes, n = 3 subjects; bone marrow, n = 8 subjects) (B). QPCR analysis of PTPγ expression in monocytes (n = 8 subjects) induced to differentiate to macrophages (moMΦ, n = 5 subjects) or dendritic cells (moDC, n = 9 subjects) in the presence or absence of LPS (n = 7 subjects) and CD40L (n = 9 subjects) (C). Statistically meaningful P values are indicated for panels A and C (calculated with unpaired t test with Welch correction). Graphs A and C are box plots in which median, 25th percentiles, 75th percentiles, minimum values, and maximum values are presented as vertical boxes and lines. In panel B, error bars represent standard deviation (SD).
Expression of PTPγ in peripheral blood, hematopoietic tissues, and differentiated monocytes. Number of copies of PTPRG mRNA per 106 copies of ACTB estimated by QPCR analysis in peripheral blood cells of individual donors (monocytes, n = 8; PBL, n = 6; PMN, n = 5; MDCs, n = 4; plasmacytoid DCs, n = 4) (A) and in primary and secondary hematopoietic tissue (spleen, n = 3 subjects; thymus, n = 2 subjects; tonsil, n = 2 subjects; lymph nodes, n = 3 subjects; bone marrow, n = 8 subjects) (B). QPCR analysis of PTPγ expression in monocytes (n = 8 subjects) induced to differentiate to macrophages (moMΦ, n = 5 subjects) or dendritic cells (moDC, n = 9 subjects) in the presence or absence of LPS (n = 7 subjects) and CD40L (n = 9 subjects) (C). Statistically meaningful P values are indicated for panels A and C (calculated with unpaired t test with Welch correction). Graphs A and C are box plots in which median, 25th percentiles, 75th percentiles, minimum values, and maximum values are presented as vertical boxes and lines. In panel B, error bars represent standard deviation (SD).
IFN-γ detection
IFN-γ was measured using a commercially available ELISA kit (Bender MedSystems, Vienna, Austria) in cell free-supernatants collected from primary MLR before the addition of [3H]-TdR. The minimum detectable dose was smaller than 1.5 pg/mL. All samples were assayed in duplicate.
Results
PTPγ mRNA expression in hematopoietic cells, tissues, and differentiated monocytes
We quantitatively measured the presence of PTPγ transcript in purified peripheral blood cells from healthy donors, where it was readily detected in monocytes (Figure 1A). The low level of expression in lymphocytes was likely caused by residual monocyte contamination (average, 5%) in the preparation, as judged by FACS analysis with anti-CD14 antibody (not shown). A similar amount of transcript was detected in MDCs and PDCs isolated from peripheral blood, whereas PTPγ transcript was nearly undetectable in Ficoll-purified polymorphonuclear cells (PMNs). Of the primary and secondary hemopoietic tissues examined (spleen, thymus, lymph nodes, tonsils, and Ficoll-purified bone marrow cells), spleen appeared to express the highest mRNA levels (Figure 1B). When monocytes were driven to differentiate into macrophages (moMΦ) or moDC by the addition of cytokines (see “Materials and methods”), PTPγ was lost in the former case but was maintained, or even increased, when monocytes differentiated into immature moDCs. Maturation induced by bacterial LPS and CD40L was associated with variable increases of PTPγ expression (Figure 1C).
PTPγ-expressing cells in spleen and other tissues
To identify the cell type expressing PTPγ in situ, immunohistochemical and double-immunofluorescence staining was performed on frozen and formalin-fixed tissue sections from lymphoid organs and normal skin.
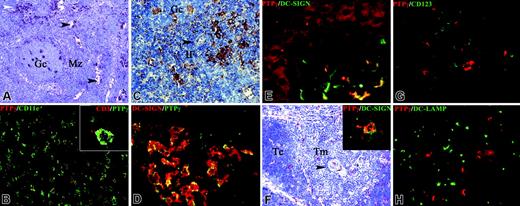
PTPγ expression in lymphoid organs and skin. Sections were obtained from spleen (A), human tonsil (B), lymph node (C-D), skin (E), and thymus (F-H). In the spleen, PTPγ+ stained tingible body macrophages of a B-cell follicle and macrophages (white arrowhead) and sinus lining cells (black arrowheads) in the red pulp (A;Gc indicates germinal center; Mz: marginal zone). Double immunofluorescence staining shows that all PTPγ+ found in the B-cell follicle coexpressed CD11c on the plasma membrane (B), and some of them clustered with CD3+ germinal center T-cells (B, inset). PTPγ+ cells with dendritic morphology are obvious in the interfollicular (IF) area, highlighted by the presence of high endothelial venules (C, black arrowhead) and in most DC-SIGN+ sinus macrophages (D, yellow cells). In normal skin, PTPγ++ positivity was diffuse in the epidermis; in the dermis, scattered PTPγ++ cells were observed, most of which showed an irregular morphology and coexpressed DC-SIGN (E, yellow cells). In normal thymus, scattered PTPγ++ cells were observed in the cortex (Tc) and in the medulla (Tm) (F), highlighted by the presence of Hassal bodies (arrowhead); no reactivity was observed on most thymocytes (F-H). PTPγ++ cells were irregular in morphology and showed abundant cytoplasm (F-H), and some of them coexpressed DC-SIGN (F, inset). PTPγ positivity was absent on CD123+ plasmacytoid dendritic cells (G) and in DC-LAMP+ mature dendritic cells (H) populating the thymic medulla. Indirect immunoperoxidase technique was applied, and nuclei were counterstained with hematoxylin (A, C, F). Double immunofluorescence was performed using FITC- and Texas red–conjugated secondary antibodies, as labeled (B, D-E, G-H).
PTPγ expression in lymphoid organs and skin. Sections were obtained from spleen (A), human tonsil (B), lymph node (C-D), skin (E), and thymus (F-H). In the spleen, PTPγ+ stained tingible body macrophages of a B-cell follicle and macrophages (white arrowhead) and sinus lining cells (black arrowheads) in the red pulp (A;Gc indicates germinal center; Mz: marginal zone). Double immunofluorescence staining shows that all PTPγ+ found in the B-cell follicle coexpressed CD11c on the plasma membrane (B), and some of them clustered with CD3+ germinal center T-cells (B, inset). PTPγ+ cells with dendritic morphology are obvious in the interfollicular (IF) area, highlighted by the presence of high endothelial venules (C, black arrowhead) and in most DC-SIGN+ sinus macrophages (D, yellow cells). In normal skin, PTPγ++ positivity was diffuse in the epidermis; in the dermis, scattered PTPγ++ cells were observed, most of which showed an irregular morphology and coexpressed DC-SIGN (E, yellow cells). In normal thymus, scattered PTPγ++ cells were observed in the cortex (Tc) and in the medulla (Tm) (F), highlighted by the presence of Hassal bodies (arrowhead); no reactivity was observed on most thymocytes (F-H). PTPγ++ cells were irregular in morphology and showed abundant cytoplasm (F-H), and some of them coexpressed DC-SIGN (F, inset). PTPγ positivity was absent on CD123+ plasmacytoid dendritic cells (G) and in DC-LAMP+ mature dendritic cells (H) populating the thymic medulla. Indirect immunoperoxidase technique was applied, and nuclei were counterstained with hematoxylin (A, C, F). Double immunofluorescence was performed using FITC- and Texas red–conjugated secondary antibodies, as labeled (B, D-E, G-H).
In normal spleen, lymph node, and tonsil, PTPγ reactivity was dominantly observed in the germinal center. Most PTPγ-expressing cells were tingible body macrophages represented by large cells with abundant cytoplasm (Figure 2A) containing phagocytosed debris (not shown). The identity of this cell population was confirmed by double immunofluorescence demonstrating coexpression of CD11c (Figure 2B) and lack of CD21/CD35 (data not shown), the latter excluding a follicular DC phenotype. Of note, double-immunofluorescence analysis revealed a preferential localization of germinal center T-lymphocytes around PTPγ+ cells (Figure 2B, inset). PTPγ expression was not restricted to the B-cell compartment. In the spleen, it was observed in macrophages and sinus-lining cells of the red pulp (Figure 2A and Figure S1, which is available on the Blood website; see the Supplemental Figure link at the top of the online article). In addition, PTPγ-positive cells with dendritic morphology were found in the interfollicular area of lymph nodes and tonsils (Figure 2C) and in nodal DC-SIGN+ sinus macrophages29 (Figure 2D). No expression of PTPγ was detected in mature interdigitating DCs of the nodal T-cell area or in plasmacytoid DCs (data not shown).
The expression of PTPγ was evaluated in normal skin by immunohistochemistry. In addition to diffuse PTPγ reactivity on epidermal keratinocytes, PTPγ-positive cells were found in the upper dermis. These cells were characterized by fusate/dendritic morphology and coexpressed DC-SIGN (Figure 2E), corresponding to dermal DCs.30,31 In normal thymus, immunohistochemistry confirmed anti-PTPγ reactivity on sparse cells in the medulla and in the cortex (Figure 2F). Positive cells displayed abundant cytoplasm and irregular morphology (Figure 2F-H) but did not coexpress pancytokeratin, thus excluding thymic epithelial cell identity (not shown). Significantly, some PTPγ+ cells coexpressed DC-SIGN (Figure 2F, inset); in contrast, no reactivity was found on the 2 major thymic DC subsets, namely CD123+ plasmacytoid DCs (Figure 2G) and DC-LAMP+ mature DCs (Figure 2H). Altogether these data indicated that PTPγ expression was retained in primary and secondary lymphoid organs by subsets of DCs and some specialized macrophages.
PTPγ expression is associated with moDC but not moMΦ differentiation: a key role for IL-4
Peripheral blood monocytes can differentiate into macrophages or DCs in vitro,32,33 and regulation of PTPγ expression during monocyte differentiation along these 2 pathways was investigated. When monocytes were cultured in vitro in the absence of exogenous cytokines, PTPγ was down-regulated within 2 hours (not shown) and remained absent in moMΦ obtained after 5-day culture. Under these experimental conditions, monocytes differentiated into large adherent cells that represented macrophagic elements, as determined by morphology and by the expression of specific surface markers (Figure 3). Activation of moMΦ by various cytokines known to regulate macrophage functions, including IL-1β, TNFα, IL-4, GM-CSF, IFNγ, IL-10, LPS, and CD40L, did not restore PTPγ expression. Furthermore, given that PTPγ-positive tingible body macrophages of the germinal center exert phagocytic activity,34 we tested the hypothesis that phagocytosis can trigger PTPγ expression in in vitro–cultured macrophages. However, the induction of phagocytosis by yeast-IgG did not lead to PTPγ expression (data not shown).
When monocytes from the same donors were differentiated into DCs in the presence of IL-4 and GM-CSF, PTPγ expression was maintained throughout the 5-day culture. Even though PTPγ expression was rapidly lost in cultured monocytes (within 2 hours; data not shown), its expression could be restored by the addition of GM-CSF and IL-4 in the medium. GM-CSF alone was ineffective, but if IL-4 was added after 3 days of culture with the sole GM-CSF, PTPγ transcript was re-expressed (Figure 3). In this case, the resultant phenotype was mixed, as suggested by the expression of the CD64 antigen. When monocytes were cultured in the absence of cytokine for 5 days, they acquired the macrophagic phenotype described. Adding IL-4 alone or in combination with GM-CSF did not induce PTPγ expression anymore (data not shown). These data indicated a critical and differentiation-dependent role for IL-4 in the induction and maintenance of PTPγ expression.
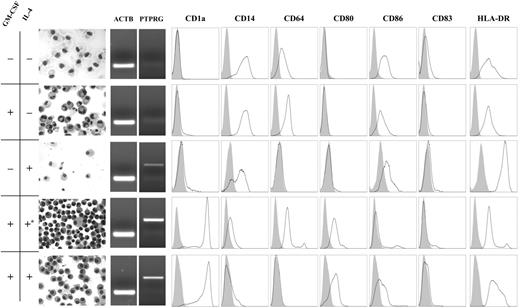
PTPγ expression is differentially modulated by IL-4 and GM-CSF. IL-4 and GM-CSF were added, either individually or in combination, at the beginning of incubation. *IL-4 was added after 3 days of GM-CSF treatment. Some of the cells were stained with May-Grunwald; the remaining cells were analyzed by cytofluorometry with a combination of myeloid markers. Large cells with large, vacuolated cytoplasm are shown in the absence of cytokines. These cells did not express PTPγ and were characterized by a macrophage phenotype (CD14+CD64+CD1a–CD80–). PTPγ began to be expressed only when IL-4 was added, but in the absence of GM-CSF the number of cells obtained in culture was decreased and the phenotype was macrophagic. When both cytokines were added, PTPγ was expressed, and both the morphology and the surface markers shifted to those of a typical DC phenotype (CD14–CD64–CD1a+CD80+). If IL-4 addition was delayed (*), the phenotype was intermediate because CD64 was still expressed and the cytoplasmic processes typical of DCs were less apparent. ACTB expression demonstrated the specificity of the signal. Results of 1 of 3 representative experiments are shown. Shaded areas represent isotype control; open areas, the specific marker.
PTPγ expression is differentially modulated by IL-4 and GM-CSF. IL-4 and GM-CSF were added, either individually or in combination, at the beginning of incubation. *IL-4 was added after 3 days of GM-CSF treatment. Some of the cells were stained with May-Grunwald; the remaining cells were analyzed by cytofluorometry with a combination of myeloid markers. Large cells with large, vacuolated cytoplasm are shown in the absence of cytokines. These cells did not express PTPγ and were characterized by a macrophage phenotype (CD14+CD64+CD1a–CD80–). PTPγ began to be expressed only when IL-4 was added, but in the absence of GM-CSF the number of cells obtained in culture was decreased and the phenotype was macrophagic. When both cytokines were added, PTPγ was expressed, and both the morphology and the surface markers shifted to those of a typical DC phenotype (CD14–CD64–CD1a+CD80+). If IL-4 addition was delayed (*), the phenotype was intermediate because CD64 was still expressed and the cytoplasmic processes typical of DCs were less apparent. ACTB expression demonstrated the specificity of the signal. Results of 1 of 3 representative experiments are shown. Shaded areas represent isotype control; open areas, the specific marker.
PTPγ colocalizes with MHC class II on DC plasma membrane
Confocal microscopy analysis of PTPγ expression was performed on moDCs using an antibody, anti-P4, that can detect PTPγ only in cells fixed and treated with detergents. It is well known that DC maturation induced by stimuli such as LPS is associated with increased MHC class II and CD83 expression on the cell surface. Analysis of immature and LPS-mature moDCs confirmed the membrane expression of PTPγ and its increased expression after LPS stimulation (Figure 4). The confocal image also suggested colocalization of PTPγ with MHC class II. The specificity of these results was supported by the observation that CD83, another cell surface marker of mature DCs, did not appear to colocalize with PTPγ (Figure 4).
PTPγ expression was increased by LPS and appeared to colocalize with MHC class II on DC plasma membrane. moDCs were left untreated (top panels) or were treated with LPS for 24 hours (bottom panels) and then were stained with control antibody (rabbit [Rbt] IgG), anti-PTPγ affinity-purified polyclonal antibody P4, followed by anti–Rbt IgG-Cy3 (red), FITC-conjugated anti–MHC class II, and anti-CD83 antibodies (green). Insets show the double staining of PTPγ-expressing cells (red) with MHC class II (third column) and CD83 (last column), both in green. PTPγ, MHC class II, and CD83 signals all increased at the cell surface on LPS treatment. MHC class II, and especially CD83, also appeared as a bright green spot in a perinuclear localization. Yellow indicates colocalization of the selected proteins that is particularly evident at the cell surface (inset, third column from left, bottom panel) corresponding to LPS-treated moDCs stained with anti–MHC class II and -PTPγ antibodies.
PTPγ expression was increased by LPS and appeared to colocalize with MHC class II on DC plasma membrane. moDCs were left untreated (top panels) or were treated with LPS for 24 hours (bottom panels) and then were stained with control antibody (rabbit [Rbt] IgG), anti-PTPγ affinity-purified polyclonal antibody P4, followed by anti–Rbt IgG-Cy3 (red), FITC-conjugated anti–MHC class II, and anti-CD83 antibodies (green). Insets show the double staining of PTPγ-expressing cells (red) with MHC class II (third column) and CD83 (last column), both in green. PTPγ, MHC class II, and CD83 signals all increased at the cell surface on LPS treatment. MHC class II, and especially CD83, also appeared as a bright green spot in a perinuclear localization. Yellow indicates colocalization of the selected proteins that is particularly evident at the cell surface (inset, third column from left, bottom panel) corresponding to LPS-treated moDCs stained with anti–MHC class II and -PTPγ antibodies.
Specificity of PTPγ expression in moMΦ and moDCs
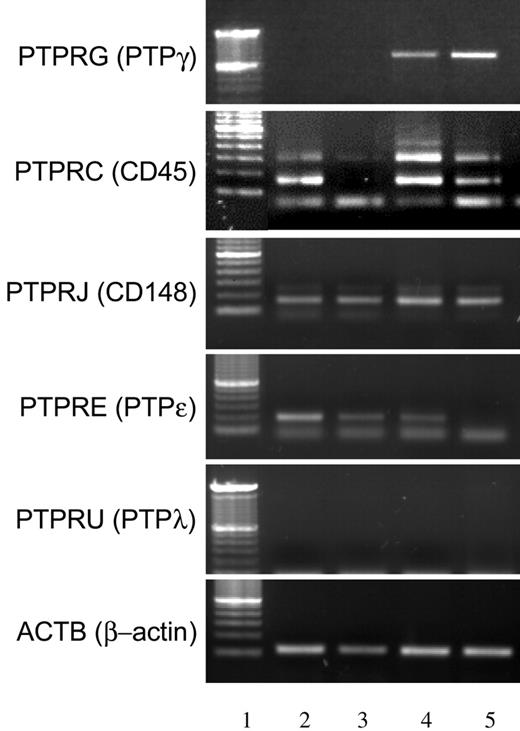
To determine the specificity of PTPγ modulation within the RPTP family, we evaluated the expression profile of 4 RPTPs known to be expressed by hematopoietic cells. PTPRC (CD45), PTPRJ (CD148), and PTPRE (PTPϵ) transcripts were found in moMΦ cells and moDCs and were down-regulated in mature DCs. PTPRU (PTPλ) was not expressed by any cell type, whereas PTPγ was always absent in moMΦ cells but was expressed and further induced by LPS treatment in moDCs (Figure 5). These data further indicated that PTPγ is an moDC-associated phosphatase and is regulated during differentiation and maturation.
PTPγ regulation is unique among other RPTPs. Expression of PTPγ mRNA in comparison with a selection of RPTPs in resting and activated moMΦ and moDCs. Lane 1, marker; lane 2, resting macrophages; lane 3, macrophages activated with 100 ng/mL LPS for 24 hours; lane 4, resting DCs; lane 5, DCs activated with 100 ng/mL LPS for 24 hours. Names of the genes analyzed appear on the left side of the picture.
PTPγ regulation is unique among other RPTPs. Expression of PTPγ mRNA in comparison with a selection of RPTPs in resting and activated moMΦ and moDCs. Lane 1, marker; lane 2, resting macrophages; lane 3, macrophages activated with 100 ng/mL LPS for 24 hours; lane 4, resting DCs; lane 5, DCs activated with 100 ng/mL LPS for 24 hours. Names of the genes analyzed appear on the left side of the picture.
PTPγ expression in specialized and reactive tissue MΦ
PTPγ expression was also investigated in tissue macrophages in the normal lung or was recruited at the site of inflammation. Alveolar macrophages appeared to strongly express PTPγ in their cytoplasm (Figure 6A); in contrast, PTPγ expression was not observed in multinucleated cells that accumulated at the site of inflammation in a patient with lymphadenitis caused by foreign body reaction (Figure 6B). In mycobacteria-induced lymphadenitis, granuloma lesions are mostly composed by a collection of specialized monocyte-derived macrophages called epithelioid cells admixed to a variable amount of T-lymphocytes. PTPγ expression was completely absent in all the cells that participated in the granuloma reaction, including epithelioid cells (Figure 6C). These data confirmed that the lack of PTPγ expression we observed in in vitro–differentiated macrophages also occurred in vivo and that PTPγ expression was a feature of selected macrophage subpopulations.
PTPγ expression correlates with a tolerogenic phenotype
We recently demonstrated that in patients with pancreatic cancer, DCs acquire a tolerogenic phenotype35 associated with a reduced capability to induce an allogeneic response and to release IFN-γ. We measured the level of PTPγ expression in moDCs derived from sex- and age-matched controls and from patients with advanced pancreatic cancer. The latter had diminished ability to induce T-cell proliferation (Figure 7A). Immature moDCs induced significantly higher IFN-γ production by T cells than did immature moDCs generated from patients (median, 1784 pg/mL; range, 1028-3425 pg/mL vs median, 1045 pg/mL; range, 521-1507 pg/mL; P = .006; Figure 7B). This feature is associated with an increased expression of PTPγ (P = .01; Figure 7C).
Discussion
PTPγ transcript is detected in a variety of human tissues, including lung, stomach, esophagus, colon, liver, spleen, and kidney.36 Aside from the reported expression in the spleen, the pattern of expression and the nature of the cells expressing PTPγ in the human hematopoietic system is unknown. A role for PTPγ in hematopoietic differentiation was described in a murine model of hematopoiesis using embryonic stem cells.9 In the present study, PTPγ mRNA expression was readily detectable in peripheral blood monocytes and, at lower levels, in circulating myeloid and plasmacytoid DCs. In addition, quantitative measurement of mRNA levels in hemopoietic and lymphoid tissues confirmed the spleen as a major PTPγ-expressing organ, followed by tonsil, lymph nodes, thymus, and Ficoll-purified bone marrow cells. Accordingly, immunohistochemical analysis of tissue sections from lymphoid organs revealed PTPγ protein expression in DCs and macrophages. In particular, PTPγ reactivity was observed in tissue-localized DCs of immature phenotype, in specialized macrophages such as alveolar and sinus macrophages, and in tingible body macrophages of the germinal center. In the spleen, PTPγ reactivity was also observed in macrophages and sinus lining cells of the red pulp, a major component of the spleen structure, thus providing a possible explanation for the abundance of PTPγ mRNA found in this organ.
In addition, a subset of PTPγ-expressing cells that exhibited DC morphology was observed in the interfollicular area of tonsil and lymph nodes. These cells have been recently characterized as immature DCs expressing DC-SIGN.37 We also found PTPγ+/DC-SIGN+ dendritic cells in the thymus and skin, in agreement with the previously reported identification of a distinct DC-SIGN+ dendritic cell subset in these organs.38 Given the selective expression of PTPγ in these myeloid cell subsets, we investigated the expression of this phosphatase and its regulation in an in vitro model of moDCs and macrophages (moMΦ). In vitro–cultured moDCs expressed PTPγ transcript and protein, and this expression was increased by maturation induced by LPS, CD40L, and TNFα. Circulating PDCs and MDCs expressed low levels of PTPγ transcript, whereas tissue PDCs and lymph node–interdigitating mature DCs did not express detectable levels of PTPγ protein. Despite the fact that MDCs and PDCs differed in their lineage of origin (for reviews, see Colonna13 and Galibert39 ), they shared PTPγ expression. Therefore, it was likely that PTPγ was finely regulated in different DC populations, mostly reflecting their degree of differentiation, function, and tissue location.
Variability of PTPγ expression in tissue macrophages. Sections were obtained from normal lung (A), a patient with foreign body reaction to oil-based contrast media (B), and a patient with mycobacterial lymphadenitis (C). In normal lung, strong PTPγ reactivity was observed in the cytoplasm of alveolar macrophages, some of them showing obvious anthracosis (arrowheads). In nodal foreign body reaction, numerous PTPγ+ sinus macrophages were present; no PTPγ reactivity was observed in numerous multinucleated giant cells surrounding lipidic drops (B, arrowheads). In mycobacterial lymphadenitis, only rare PTPγ cells were found outside the granuloma, and no staining was observed in epithelioid macrophages (C, asterisks). Indirect immunoperoxidase technique was applied, and nuclei were counterstained with hematoxylin.
Variability of PTPγ expression in tissue macrophages. Sections were obtained from normal lung (A), a patient with foreign body reaction to oil-based contrast media (B), and a patient with mycobacterial lymphadenitis (C). In normal lung, strong PTPγ reactivity was observed in the cytoplasm of alveolar macrophages, some of them showing obvious anthracosis (arrowheads). In nodal foreign body reaction, numerous PTPγ+ sinus macrophages were present; no PTPγ reactivity was observed in numerous multinucleated giant cells surrounding lipidic drops (B, arrowheads). In mycobacterial lymphadenitis, only rare PTPγ cells were found outside the granuloma, and no staining was observed in epithelioid macrophages (C, asterisks). Indirect immunoperoxidase technique was applied, and nuclei were counterstained with hematoxylin.
Tolerogenic phenotype of moDCs derived from patients with pancreatic cancer is associated with decreased PTPγ expression. (A) Allostimulatory function (MLR) of immature moDCs generated from patients with advanced or metastatic pancreatic carcinoma (n = 24, •) compared with control immature moDCs (n = 15, ○). Results are expressed as mean ± SD of [3H-TdR] uptake after 5 days of MLR culture in triplicate and show a reduced capability to stimulate T-cell proliferation by the former. CPM for T cells alone was 3963 ± 984. (B) IFN-γ production by allogeneic T cells stimulated by immature moDC from healthy donors (n = 15) and patients with advanced or metastatic pancreatic carcinoma (n = 15). Cytokine levels were detected by ELISA in cell-free supernatants collected from primary MLR before the addition of 3H-TdR. (C) PTPγ expression was increased in patients with metastatic pancreatic carcinoma (n = 9) compared with healthy donors (n = 11). (B-C) Median, 25th percentiles, 75th percentiles, minimum values, and maximum values are presented as vertical boxes and lines.
Tolerogenic phenotype of moDCs derived from patients with pancreatic cancer is associated with decreased PTPγ expression. (A) Allostimulatory function (MLR) of immature moDCs generated from patients with advanced or metastatic pancreatic carcinoma (n = 24, •) compared with control immature moDCs (n = 15, ○). Results are expressed as mean ± SD of [3H-TdR] uptake after 5 days of MLR culture in triplicate and show a reduced capability to stimulate T-cell proliferation by the former. CPM for T cells alone was 3963 ± 984. (B) IFN-γ production by allogeneic T cells stimulated by immature moDC from healthy donors (n = 15) and patients with advanced or metastatic pancreatic carcinoma (n = 15). Cytokine levels were detected by ELISA in cell-free supernatants collected from primary MLR before the addition of 3H-TdR. (C) PTPγ expression was increased in patients with metastatic pancreatic carcinoma (n = 9) compared with healthy donors (n = 11). (B-C) Median, 25th percentiles, 75th percentiles, minimum values, and maximum values are presented as vertical boxes and lines.
In addition to DCs, we observed PTPγ expression in lymph node sinus macrophages. Although these cells are considered macrophages, the sinus conduit system in mice is known to contain both CD11b+/CD11c+ and CD11b+/CD11c– mononuclear cells.40 Interestingly, some authors proposed to consider marginal sinus macrophages “sinus DCs” based on the selective expression of the Ki-M9 antigen.41 Lung alveolar MΦ also expressed PTPγ. It is of note that PTPγ+ DCs coexpressed DC-SIGN, a feature shared by sinus and alveolar MΦ,38 suggesting that the PTPγ+/DC-SIGN+ phenotype may identify specific subsets of tissue macrophages that share functional features with DCs in terms of high-level antigen presentation.
Tingible body macrophages are CD11c+ myeloid cells with poorly characterized function. Their main role in germinal center reaction is related to the scavenger function of apoptotic germinal center B cells (for reviews, see MacLennan42 and Smith et al43 ). We found that they expressed PTPγ and physically interacted with germinal center T cells. Taken together, these data suggest a more complex role of these cells in the generation of the germinal center reaction.
In contrast, in the interfollicular area, PTPγ+ DCs can interact with and instruct a mixture of lymphoid cells recently immigrated from the peripheral blood through the high endothelial venules. In keeping with this hypothesis, the specific colocalization of PTPγ and MHC class II, in association with its tyrosine phosphatase activity, might suggest its involvement in the regulation of the “immunologic synapse.”44,45
Little is known about factors that modulate PTPγ mRNA expression. Human astrocytoma cells express this phosphatase under the influence of IL-1α/IL-1β or TNFα.17β-Estradiol, which down-regulates PTPγ expression in normal cells and breast cancer cells, is the only other molecule known to modulate its expression.46 Based on the data reported here and on the fact that cytokines represent important factors for myeloid cell differentiation/activation, we attempted to identify in vitro conditions that could modulate PTPγ expression. IL-4 is a critical cytokine that prevents PTPγ down-modulation induced by in vitro culture of monocytes. Under these experimental conditions, PTPγ expression is up-regulated by proinflammatory stimuli such as LPS, CD40L, and TNFα. We observed that specialized macrophage populations expressed PTPγ in vivo, and we wondered whether PTPγ could also be induced in moMΦ in vitro. However, moMΦ failed to express PTPγ in vitro in any of the experimental conditions investigated, such as IL-1β, TNFα, IL-4, IFNγ, IL-10, and GM-CSF, alone or in combination. The same cells did not express PTPγ after LPS and CD40L stimulation or after the ingestion of IgG-coated yeast particles. These results mimic the absence of PTPγ expression by foreign body–reactive MΦ and by epithelioid cells from granulomatous lymphadenitis in vivo, which are assumed to derive from circulating monocytes.47-49
In addition, it must be considered that PTPγ expression in moDC and moMΦ is unique within the members of the transmembrane PTP family expressed in hemopoietic cells. It also appeared to be the only one whose expression was increased after maturation.
PTPγ can be considered an in vitro marker of the DC differentiation pathway, whereas in vivo its expression is present in a subset of immature DC SIGN+ DC and in certain subsets of specialized macrophages, namely those localized in the lung, germinal center, and sinus conduit in the lymph node. In parallel with the in vitro data, in vivo inflammatory macrophages do not express PTPγ. Interestingly, IL-4, the key cytokine for the expression of PTPγ in vitro, is also known to increase the expression of DC-SIGN.50 Given that PTPγ expression in vivo is restricted to certain subsets of DCs and macrophages, it is likely that this protein might play a role in determining the functional heterogeneity that characterizes these populations. In keeping with this hypothesis, the results of our investigation strongly suggest that pancreatic carcinoma–associated cytokines lead to the development of a predominant tolerogenic DC2 phenotype, characterized by low expression levels of costimulatory molecules35 and poor T-cell allostimulatory capacity. We observed that these features associated with higher PTPγ expression. DC capability to induce tolerance has been demonstrated in immature and mature DCs.51 Therefore, the evidence that PTPγ expression is observed in immature DCs in vitro and in vivo, is induced by LPS and CD40L in moDCs, and characterizes a subset of tolerogenic moDCs in cancer patients is in keeping with the possibility that PTPγ levels could represent a crucial event that defines or modulates the function of specific DCs and other myeloid cells.
Future identification of PTPγ ligand and better understanding of the role of this protein in cell function will contribute to our understanding of the biological role of DC and macrophage subpopulations.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
D.L., W.V., and M.V. equally contributed to this study. Daniele Lissandrini died on September 4, 2004.
Prepublished online as Blood First Edition Paper, August 8, 2006; DOI 10.1182/blood-2006-05-024257.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
This work was supported by grants from Consorzio Studi Universitari, Verona, Italy; Fondazione Cariverona (Bando 2003); AIRC (Associazione Italiana per la Ricerca sul Cancro); Fondazione Compagnia di San Paolo, Torino, Italy; MIUR (Ministero dell'Istruzione Università e Ricerca) grant 40F.37 “Programma nazionale di Ricerca sull'AIDS” (Istituto Superiore di Sanità, 2004); and Fondazione Beretta (Brescia, Italy).
The authors thank Brian Weaver (Department of Pathology and Immunology, Washington University, St Louis, MO) and Martin Pelletier (Department of Pathology, University of Verona) for careful review of the manuscript.
This article is dedicated to Daniele Lissandrini, a serious scientist who, despite troubles, never lost his interest in life science, and a deeply caring person with an irrepressible sense of humor.


![Figure 4. PTPγ expression was increased by LPS and appeared to colocalize with MHC class II on DC plasma membrane. moDCs were left untreated (top panels) or were treated with LPS for 24 hours (bottom panels) and then were stained with control antibody (rabbit [Rbt] IgG), anti-PTPγ affinity-purified polyclonal antibody P4, followed by anti–Rbt IgG-Cy3 (red), FITC-conjugated anti–MHC class II, and anti-CD83 antibodies (green). Insets show the double staining of PTPγ-expressing cells (red) with MHC class II (third column) and CD83 (last column), both in green. PTPγ, MHC class II, and CD83 signals all increased at the cell surface on LPS treatment. MHC class II, and especially CD83, also appeared as a bright green spot in a perinuclear localization. Yellow indicates colocalization of the selected proteins that is particularly evident at the cell surface (inset, third column from left, bottom panel) corresponding to LPS-treated moDCs stained with anti–MHC class II and -PTPγ antibodies.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/13/10.1182_blood-2006-05-024257/7/m_zh80240605240004.jpeg?Expires=1769141332&Signature=yYRoF1ea9wr3fV4melpLJ33cIDqv7De1aeJq49Pvb9cTKZnEO64pxL2~6ksgUUQ4az6z4AAPku2Nj697KLLsl2lVXzzRL2tSRMXUL61HRX~dH~84w1KeMfpEfhacGTdqFQpt7Xoc6cNcu7FP2osFwv2CycNjlhYxpAu7NBy~4OonazinzYIW645KoU2hRcPC9Yy3OU7TCL0SxtI-DxIF2hc3VReIquN57~WAdHETy8H-YiseCYbDC-DlYyNTSBFz3cXEDt2ezcaBesZY0XigkKAZgt3ZLlVdkcQ-gpPRsM9lXmRANoNalLy6zE8OB40d8eEspq8q5q9bp0xYrw1c~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 7. Tolerogenic phenotype of moDCs derived from patients with pancreatic cancer is associated with decreased PTPγ expression. (A) Allostimulatory function (MLR) of immature moDCs generated from patients with advanced or metastatic pancreatic carcinoma (n = 24, •) compared with control immature moDCs (n = 15, ○). Results are expressed as mean ± SD of [3H-TdR] uptake after 5 days of MLR culture in triplicate and show a reduced capability to stimulate T-cell proliferation by the former. CPM for T cells alone was 3963 ± 984. (B) IFN-γ production by allogeneic T cells stimulated by immature moDC from healthy donors (n = 15) and patients with advanced or metastatic pancreatic carcinoma (n = 15). Cytokine levels were detected by ELISA in cell-free supernatants collected from primary MLR before the addition of 3H-TdR. (C) PTPγ expression was increased in patients with metastatic pancreatic carcinoma (n = 9) compared with healthy donors (n = 11). (B-C) Median, 25th percentiles, 75th percentiles, minimum values, and maximum values are presented as vertical boxes and lines.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/13/10.1182_blood-2006-05-024257/7/m_zh80240605240007.jpeg?Expires=1769141332&Signature=aP7ktKEm4lXq1LyDDl6NFbjahO4PVJgf0zHjIb5owFqzNCC3cQm63lpstRPtu2lMlNQd3Rrqqa4SJnUzUbX4v9uQ7KtQ3vAsPLsnYUzq1DM3XrNU~fKANkOmYLZj3L~sRvyVvUIyZzqIm5-gKGJGIFuj-yVQwjtj4nGYpyAY6OpMtJtpfA0T4ySVyKcmL8~sl6r~rnpRkSZG8aD-65mbHbYDY-TGEBhUl2CT~L3iyYMGY0jvKCs7M6IQ2dtYT7Z23~cNdlqpryMdM-jq-1m8O~wfXNbNQNLxgjXzdcYoLilqu6W6U2stbAuljepEDdwE6K~iU6ppoO5ZRM18YtfbAA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
