Comment on Smith et al, page 653
Enforced transgenic expression of c-MYC causes monocytic neoplasms in heterozygous transgenic mice but T-cell lymphomas in homozygous transgenic mice, demonstrating that finely tuned thresholds of MYC expression induce neoplastic transformation in different blood cell types.
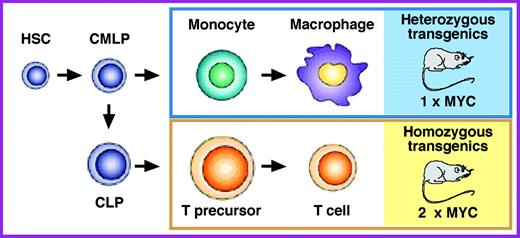
MYC is an important oncoprotein in a wide range of hematopoietic neoplasms in human beings. However, the precise levels of MYC that induce the malignant transformation of different types of blood cells have not yet been determined. Postulating that mouse models of widespread deregulated MYC expression in the hematopoietic system may be helpful to remedy this shortcoming, Suzanne Cory and her associates at the Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia, took advantage of regulatory elements of the Vav gene to express MYC throughout the hematopoietic system in transgenic mice. In this issue of Blood, they report on 3 newly developed mouse strains that exhibit a striking dependence of onset, incidence, and types of hematopoietic neoplasms on MYC expression levels in blood cells. Smith and colleagues show that in these strains high MYC leads to early-onset aggressive T-cell lymphoma, whereas low MYC causes mainly late-onset monocytic neoplasia. Comparing heterozygous and homozygous transgenic offspring from the same strain leads the authors to the intriguing observation that, everything else being equal, a mere doubling of the MYC level results in a dramatic switch in tumor phenotype from monocytic to Tlymphocytic neoplasms (see the figure). Smith and colleagues conclude that blood cells exhibit distinct, sharp thresholds for MYC-driven oncogenesis, possibly because MYC levels determine the likelihood with which tumor precursors acquire collaborating oncogenic mutations.
The findings in the newly developed MYC transgenic mice have important implications for human cancers in which the fine-tuning of the levels of deregulated MYC is increasingly recognized as a mechanism of tumor initiation and progression. A widely known example along this line is the occurrence of certain hot-spot mutations in the MYC gene in Burkitt lymphoma, which result in elevated stability of the MYC protein in the tumor cells. Fully appreciating the complexity of the cellular signaling pathways that affect the turnover and activity of MYC in blood cells, Smith at al propose to take advantage of their new MYC transgenics to elucidate these pathways in greater depth. Since a modest 2-fold decrease in the level of MYC is likely to reduce, if not abrogate, T-cell lymphoma in the homozygous MYC transgenics depicted in the figure, the authors further propose that these mice may prove valuable for facilitating the design and testing of new therapeutic approaches to deregulated MYC. Clearly, evaluating the efficacy of emerging therapeutic agents that inhibit MYC expression, disrupt MYC's interaction with other proteins, or block MYC target function is a much more difficult task in clinical trials than in genetically and environmentally defined preclinical model systems afforded by transgenic mice. As with all good studies, the present Blood paper raises many new questions. Among these is whether monocytes and immature T lymphocytes are the direct targets of MYC-induced cell transformation (see the figure, right), or whether MYC acts at an earlier stage of blood-cell development, such as the lineage-committed precursors or the hematopoietic stem cell depicted at the left of the figure. ▪FIG1
MYC levels determine the types of hematopoietic neoplasms that develop in mice carrying 1 or 2 copies of the same Vav-driven MYC transgene. Heterozygous MYC transgenic mice are prone to late-onset monocytic neoplasms (blue box), whereas homozygous MYC transgenic mice, which express twice as much MYC (2 × MYC) as their heterozygous counterparts do (1 × MYC), develop early-onset T-cell lymphomas (yellow box). HSC indicates hematopoietic stem cell; CMLP, common myelolymphoid progenitor; and CLP, common lymphoid progenitor.
MYC levels determine the types of hematopoietic neoplasms that develop in mice carrying 1 or 2 copies of the same Vav-driven MYC transgene. Heterozygous MYC transgenic mice are prone to late-onset monocytic neoplasms (blue box), whereas homozygous MYC transgenic mice, which express twice as much MYC (2 × MYC) as their heterozygous counterparts do (1 × MYC), develop early-onset T-cell lymphomas (yellow box). HSC indicates hematopoietic stem cell; CMLP, common myelolymphoid progenitor; and CLP, common lymphoid progenitor.