CD30 is a member of the tumor necrosis factor receptor family. Overexpression of CD30 on some neoplasms versus its limited expression on normal tissues makes this receptor a promising target for antibody-based therapy. Anaplastic large-cell lymphoma (ALCL) represents a heterogeneous group of aggressive non-Hodgkin lymphomas characterized by the strong expression of CD30. We investigated the therapeutic efficacy of HeFi-1, a mouse IgG1 monoclonal antibody, which recognizes the ligand-binding site on CD30, and humanized anti-Tac antibody (daclizumab), which recognizes CD25, in a murine model of human ALCL. The ALCL model was established by intravenous injection of karpas299 cells into nonobese diabetic/severe combined immuno-deficient (SCID/NOD) wild-type or SCID/NOD Fc receptor common γ chain–deficient (FcRγ–/–) mice. HeFi-1, given at a dose of 100 μg weekly for 4 weeks, significantly prolonged survival of the ALCL-bearing SCID/NOD wild-type and SCID/NOD FcRγ–/– mice (P < .01) as compared with the control groups. In vitro studies showed that HeFi-1 inhibited the proliferation of karpas299 cells, whereas daclizumab did not inhibit cell proliferation. We demonstrated that the expression of FcRγ on polymorphonuclear leukocytes and monocytes was not required for HeFi-1–mediated tumor growth inhibition in vivo, although it was required for daclizumab.
Introduction
CD30 is a member of the tumor necrosis factor (TNF) receptor family, which includes TNF-R1, TNF-R2, Fas-R, CD40, CD27, and TRAIL-R.1 Increased expression of CD30 is observed on some neoplasms, including Hodgkin disease, anaplastic large-cell lymphoma (ALCL), mediastinal B-cell lymphoma, embryonal carcinoma, seminoma, and mesothelioma.2-7 In contrast, its expression in normal tissues is limited to activated T cells, activated B cells, select thymocytes, and some vascular beds.3 This expression on neoplasms versus its limited expression on normal tissues makes it a promising target for antibody-based therapy.
HeFi-1 is a murine IgG1, which recognizes the ligand-binding site on CD30. ALCL represents a heterogeneous group of aggressive non-Hodgkin lymphomas characterized by the strong expression of CD30 and a frequent involvement of the t(2;5) chromosomal translocation.8 Despite responsiveness to chemotherapy, approximately one third of the patients with ALCL die regardless of intensive chemotherapy. Thus, alternative clinical approaches need to be developed. Anti-CD30 antibody-based immunotherapy has been investigated in vitro and in vivo.9-12 CD30-mediated signal transduction is capable of promoting cell proliferation and cell survival as well as antiproliferative effects and cell death depending on cell type and co-stimulatory effects.13 CD30 activation was reported to induce cell growth inhibition and apoptosis with some but not all ALCL cells.9,10,14 In particular, the treatment of ALCL-derived cell lines, karpas299 and Michel, with 2 antibodies (M44, HeFi-1) that recognize the ligand-binding site of CD30, led to a significant reduction of cell viability.10 Preclinical studies showed that overall survival and disease-free survival of severe combined immunodeficient (SCID) mice bearing extensive metastases of karpas299 were significantly enhanced by anti-CD30 treatment.12
In the present study, we investigated the efficacy of HeFi-1 in a murine model of human ALCL. We were particularly interested in the mechanism underlying the inhibition of the tumor growth mediated by HeFi-1 on ALCL. The anti-CD30 monoclonal antibody, HeFi-1, unlike the anti-CD25 antibody, showed the therapeutic efficacy in an ALCL model in both nonobese diabetic/severe combined immunodeficient (SCID/NOD) wild-type and SCID/NOD Fc receptor common γ chain–deficient (FcRγ–/–) mice, suggesting that expression of the receptor FcRγIII is not required for the effective action of this antibody in this mouse lymphoma model.
Materials and methods
Monoclonal antibodies
HeFi-1, which is a mouse IgG1 directed toward the ligand-binding site on CD30, was provided by the Biological Response Modifiers Program, National Cancer Institute–Frederick Cancer Research Center. The humanized anti-Tac antibody (daclizumab), which recognizes CD25 (IL-2Rα), was obtained from Hoffmann–La Roche (Nutley, NJ). Murine anti-Tac (MAT), which recognizes the same epitope as daclizumab, was produced as described previously.15,16 B3, a mouse IgG1 reacting with a carbohydrate epitope found on the Ley and the polyfucosylated-Lex antigens,17 was used as an isotype-matched control antibody that did not bind to karpas299 cells.
Tumor cell line and mouse model
Karpas299, a human anaplastic large-cell lymphoma (ALCL) cell line, expresses both CD30 and CD25 on the cell surfaces. Karpas299 cells were maintained in RPMI 1640 (Invitrogen, Carlsbad, CA) containing 10% heat-inactivated fetal bovine serum (Gemini Bio-Products, Woodland, CA), 100 U/mL penicillin, and 100 μg/mL streptomycin in an atmosphere containing 5% CO2. SCID/NOD mice were purchased from Jackson Laboratories (Bar Harbor, ME), and SCID/NOD Fc receptor common γ chain–deficient (FcRγ–/–) mice were generated in the laboratory of Jeffrey Ravetch (Rockefeller University, New York, NY). The ALCL model was established by intravenous injection of 1 × 107 karpas299 cells (200 μL) into SCID/NOD wild-type or SCID/NOD FcRγ–/– mice. The therapy experiments were performed on the ALCL lymphoma–bearing mice at day 7 after karpas299 cell injection. All of the mice used in this study were 8 to 10 weeks old, and the ages of the mice in the different groups in the same experiment were matched. All animal experiments were performed in accordance with National Institutes of Health Animal Care and Use Committee guidelines.
Expression of CD30 and CD25 on karpas299 cell surfaces
The expression of CD30 and CD25 on karpas299 cell surfaces was analyzed by flow cytometry. Aliquots of 1 × 106 karpas299 cells were incubated with the primary antibody, HeFi-1, or MAT or isotype control antibodies (1 μg/100 μL) on ice for 30 minutes. The cells were washed and then stained with a fluorescein isothiocyanate (FITC)–labeled goat anti–mouse IgG antibody (SouthernBiotech, Birmingham, AL). After washing, the cells were analyzed for the expression of CD30 and CD25 using FACScan flow cytometry (Becton Dickinson, San Jose, CA).
Immunoreactivity of radiolabeled HeFi-1 and daclizumab
Conjugation of HeFi-1 and daclizumab to CHX-A″ was performed as previously described.18 HeFi-1-CHX-A″, daclizumab-CHX-A″, and B3-CHX-A″ were labeled with 111In at specific activities of 74-111 kBq/μg as described previously.19 For the immunoreactivity experiment, 111In-HeFi-1, 111In-daclizumab, or 111In-B3 (10 ng/100 μL) was incubated with an increasing number of karpas299 cells (1 × 104–5 × 106) with or without unlabeled HeFi-1 or daclizumab (25 μg/tube) inhibition at 4°C for 1 hour. After centrifugation, the supernatant was aspirated, and the radioactivity bound to the cells was quantitated in a γ counter (Wallac, Turku, Finland).
Proliferation assay
Karpas299 cells were resuspended at a concentration of 1 × 105 cells/mL. Aliquots of 1 × 104 cells were seeded in 96-well culture plates and incubated with medium alone or with antibodies at a concentration of 20 μg/mL at 37°C. The cells were pulsed after 18 or 42 hours of culture for 6 hours with 1 μCi (0.037 MBq) [3H]thymidine. Then, the cells were harvested with a 96-well harvester (Tomtec, Hamden, CT) and counted in a β counter (Wallac). The assay was performed in triplicate on 3 occasions.
Cell-cycle analysis
Karpas299 cells were collected and washed with phosphate buffered saline (PBS) containing 1% bovine serum albumin (PBS/BSA) and 0.02% sodium azide after incubation with medium alone or with antibodies (20 μg/mL) for 48 hours in 6-well culture plates (4 × 105 cells/4 mL/well). The cells were fixed with 70% ethanol on ice for 20 minutes. The cells then were incubated with 100 μL (50 μg/mL) of DNase-free RNase (Roche Applied Science, Indianapolis, IN) at 37°C for 30 minutes and stained with 300 μL (50 μg/mL) propidium iodide (Roche Applied Science). The DNA content was measured by a FACScan flow cytometer (Becton Dickinson), and cell-cycle analysis was performed using Modfit software (Verity, Topsham, ME).
Therapy study
For the evaluation of therapeutic efficacy of the anti-CD30 antibody HeFi-1 and the anti-CD25 antibody daclizumab, groups of karpas299 lymphoma-bearing SCID/NOD wild-type mice were injected with 100 μg HeFi-1, daclizumab, or 200 μL PBS intravenously weekly for 4 weeks. To define the mechanism of action of the antibodies, the second therapeutic study was performed in karpas299-bearing SCID/NOD wild-type and SCID/NOD FcRγ–/– mice using the same dose schedule as that used in the first experiment. Throughout the experiment, tumor progression was monitored by body weight and/or Kaplan Meier analysis of survival of the karpas299-bearing mice. We repeated the therapeutic study once using HeFi-1 and daclizumab at the same dose schedule and with the same tumor model in both SCID/NOD wild-type and SCID/NOD FcRγ–/– mice.
Measurement of ALCL lymphoma growth
The growth of the ALCL lymphoma was confirmed by pathologic examination (Pathology Laboratory, National Cancer Institute, Frederick Cancer Research and Development Center, Frederick, MD). It was reported that there were elevated serum sIL-2Rα levels in patients with ALCL, and the sIL-2Rα levels correlated well with serum-soluble CD30, which was associated with a worse outcome.20 Karpas299 cells are CD25 positive, and serum sIL-2Rα levels of the karpas299-bearing mice may serve as a surrogate marker of the tumor burden. Measurements of the serum concentrations of sIL-2Rα and/or soluble human β-2-microglobulin (β2μ) were performed using an enzyme-linked immunosorbent assay (ELISA). The ELISA kits were purchased from R&D Systems (Minneapolis, MN). The ELISAs were performed as indicated in the manufacturer's kit inserts. The body weight and/or survival of the karpas299 lymphoma-bearing mice were monitored throughout the experiments.
Statistical analysis
The serum levels of sIL-2Rα and the body weight of the karpas299 lymphoma-bearing mice were analyzed at different time points for the different treatment groups using the Student t test for unpaired data. Statistical significance of differences in survival of the mice in different groups was determined by the log-rank test using StatView program (Abacus Concepts, Berkeley, CA).
Results
Immunoreactivity of radiolabeled HeFi-1 and daclizumab
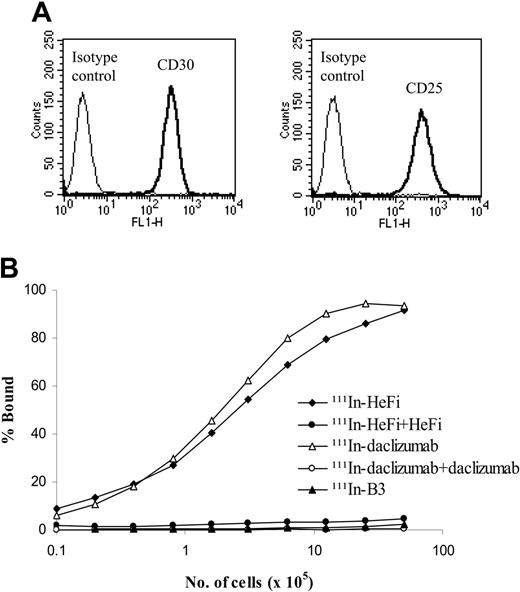
HeFi-1 and daclizumab target distinct receptors, CD30 and CD25, respectively, that are expressed on the karpas299 cell surfaces (Figure 1A). Both 111In-HeFi-1 and 111In-daclizumab bound to karpas299 cells specifically, with a maximum binding of more than 90% of the added radiolabeled antibody (Figure 1B).
Antiproliferation effect of HeFi-1
Karpas299 cells were treated with medium alone or with the antibodies at a concentration of 20 μg/mL for 48 hours. HeFi-1 inhibited the proliferation of the cells by 40% when compared with cells treated with medium alone or the isotype control, B3 antibody, whereas daclizumab did not inhibit the proliferation of the cells (Figure 2A).
Expression of CD30 and CD25 on karpas299 cell surfaces evaluated by flow cytometric analysis and immunoreactivities of 111In-HeFi-1 and 111Indaclizumab with karpas299 cells. (A) Karpas299 cells strongly express both CD30 and CD25 on the cell surfaces. (B) Both 111In-HeFi-1 and 111In-daclizumab bound to karpas299 cells specifically, with maximum binding percentage more than 90%. 111In-B3 did not bind to karpas299 cells. Both experiments were repeated once.
Expression of CD30 and CD25 on karpas299 cell surfaces evaluated by flow cytometric analysis and immunoreactivities of 111In-HeFi-1 and 111Indaclizumab with karpas299 cells. (A) Karpas299 cells strongly express both CD30 and CD25 on the cell surfaces. (B) Both 111In-HeFi-1 and 111In-daclizumab bound to karpas299 cells specifically, with maximum binding percentage more than 90%. 111In-B3 did not bind to karpas299 cells. Both experiments were repeated once.
HeFi-1–mediated cell-cycle arrest
To examine which mechanism plays a major role in inhibiting the proliferation of karpas299 cells, the cells were treated with the antibodies for 48 hours, and then apoptosis and cell-cycle analyses were performed. HeFi-1 inhibited the cell growth mainly by causing cell-cycle arrest at G1 phase (Figure 2B). In contrast, daclizumab did not show any affect on the cell cycle (Figure 2B).
ALCL lymphoma model
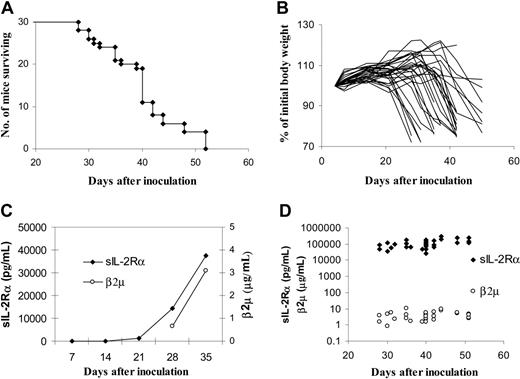
SCID/NOD mice were used to establish this model because the mice lack functional B, T, and natural killer (NK) cells. After intravenous injection of 1 × 107 karpas299 cells, the animals began to die around 4 weeks, and all of the karpas299-bearing mice succumbed to the lymphoma within 2 months (Figure 3A). Autopsy showed that numerous tumor nodules developed around the neck and head, as well as some in the peritoneal cavity, and some of the mice developed tumors in the eyes and cranium. Pathologic examination showed that there was widespread tissue distribution of the lymphoma, including bone marrow, lymph nodes, brain, kidney, eye, and cranium. The body weight of the karpas299-lymphoma bearing mice decreased with disease progression (Figure 3B). Although the serum levels of sIL-2Rα and β2μ were not detectable at an early stage of disease, these increased over time (Figure 3C) and reached levels of 25 000-300 000 pm/mL and 1 to 10 ng/mL, respectively, immediately before death (Figure 3D).
Therapeutic study with HeFi-1 and daclizumab
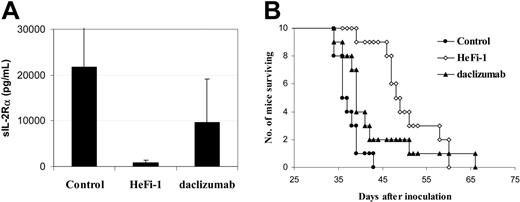
In the therapeutic study, HeFi-1 or daclizumab was injected intravenously weekly for 4 weeks at a dose of 100 μg. Therapeutic effects in SCID/NOD wild-type mice bearing karpas299 lymphoma were demonstrated by their effects on the serum levels of sIL-2Rα (Figure 4A), a surrogate tumor marker that was indicative of the tumor load of karpas299 lymphoma in the murine model and by the survival of the lymphoma-bearing mice (Figure 4B). When compared with the serum concentration of sIL-2Rα in the control group, on day 21 after therapy, there was a reduction of sIL-2Rα levels in the daclizumab treatment group, although it did not achieve statistical significance (Figure 4A). In addition, there was a significant reduction of sIL-2Rα levels in the HeFi-1 treatment group (Figure 4A; P < .001). Furthermore, there was a significant prolongation of the survival of the mice in the treatment groups receiving either HeFi-1 or daclizumab when compared with that in the control group (Figure 4B; P < .05). The mean survival duration of the control group was 37.2 days, whereas it was prolonged to 42.9 days in the daclizumab group and 50.5 days in the HeFi-1 group (Figure 4B).
FcRγ expression is required for effective action of daclizumab, but not for that of HeFi-1
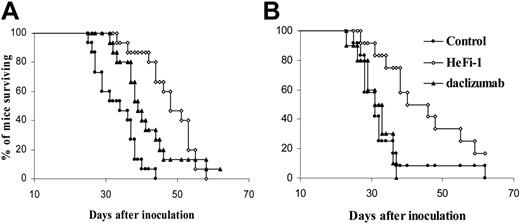
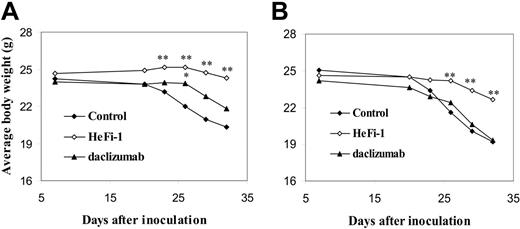
Both HeFi-1 and daclizumab demonstrated therapeutic efficacy in the karpas299 lymphoma model, although HeFi-1 was more effective than daclizumab (Figure 4A,B). To define the mechanism of action of the antibodies, another therapeutic study was performed in the karpas299-bearing SCID/NOD wild-type and SCID/NOD FcRγ–/– mice to determine whether Fc-dependent cellular cytotoxicity is involved in the mechanism of the tumor killing by HeFi-1 and daclizumab. The therapeutic efficacy of daclizumab in the lymphoma-bearing SCID/NOD wild-type mice was repeated. Daclizumab, given intravenously weekly for 4 weeks at a dose of 100 μg, prolonged the survival of the karpas299 lymphoma-bearing SCID/NOD wild-type mice significantly as compared with the control group (Figure 5A; P < .01). The mean survival duration of the control group was 26.2 days, whereas it was prolonged to 34.4 days in the daclizumab group. However, daclizumab, given at the same dose schedule as that in the karpas299 lymphoma-bearing SCID/NOD wild-type mice, did not show any therapeutic efficacy in SCID/NOD FcRγ–/– mice bearing the same quantity of the karpas299 cells. There were no statistical differences in the survival and the body weight of the mice observed between the daclizumab treatment group and the control group in SCID/NOD FcRγ–/– mice bearing karpas299 lymphoma (Figures 5B,6B). In contrast, HeFi-1 showed therapeutic efficacy in both SCID/NOD wild-type and SCID/NOD FcRγ–/– mice bearing karpas299 lymphoma as seen by the survival (Figure 5A,B) and the body weight (Figure 6A,B). There were significant differences in the body weight between the HeFi-1 treatment group and the control group in both SCID/NOD wild-type and SCID/NOD FcRγ–/– mice bearing karpas299 lymphoma (Figure 6A,B; P < .001). Furthermore, there were significant prolongations of survival of both SCID/NOD wild-type and SCID/NOD FcRγ–/– mice bearing karpas299 lymphoma in HeFi-1 treatment groups when compared with those in the control groups (Figure 5A,B; P < .01). The mean survival durations of the control groups were 26.2 and 26.1 days in SCID/NOD wild-type and SCID/NOD FcRγ–/– mice, respectively, whereas they were prolonged to 40.9 and 33.4 days in the HeFi-1 treatment groups. Similar results were observed in a repeat therapeutic study. The results of a pharmacokinetic study (data not shown) that indicated comparable monoclonal antibody survivals in both SCID/NOD wild-type and SCID/NOD FcRγ–/– mice excluded a contribution, provided by different concentrations of the antibodies in the blood, to any different therapeutic efficacies observed with SCID/NOD wild-type mice as compared to SCID/NOD FcRγ–/– mice. Thus, FcRγ expression is required for effective action of daclizumab but not for that of HeFi-1 in this ALCL model.
The effect of HeFi-1 and daclizumab on the proliferation and cell cycle of karpas299 cells in vitro. (A) Karpas299 cells were incubated with medium alone or with antibodies (20 μg/mL) at 37°C for 42 hours and pulsed with 1 μCi (0.037 MBq) [3H]thymidine for 6 hours. The cells then were harvested and counted. The data represent mean ± SD of triplicates and are representative of 3 experiments. HeFi-1 inhibited the proliferation of karpas299 cells, whereas daclizumab did not inhibit the proliferation. CPM indicates counts per minute. (B) The cells were harvested for flow cytometric analysis after incubating with medium alone or with antibodies (20 μg/mL) for 48 hours. Percentage of apoptotic cells with sub-G1 DNA content (M1 phase) was indicated in the histograms, and the percentages of cells in different phases of the cell cycle were analyzed using Modfit software. Similar results were obtained in 3 independent experiments. HeFi-1 caused cell-cycle arrest as defined by the reduced percentage of the cells in S phase and the increased percentage in G1 phase when compared with those of the cells treated with medium alone or B3 antibody. The daclizumab did not show induction of apoptosis or cell-cycle arrest.
The effect of HeFi-1 and daclizumab on the proliferation and cell cycle of karpas299 cells in vitro. (A) Karpas299 cells were incubated with medium alone or with antibodies (20 μg/mL) at 37°C for 42 hours and pulsed with 1 μCi (0.037 MBq) [3H]thymidine for 6 hours. The cells then were harvested and counted. The data represent mean ± SD of triplicates and are representative of 3 experiments. HeFi-1 inhibited the proliferation of karpas299 cells, whereas daclizumab did not inhibit the proliferation. CPM indicates counts per minute. (B) The cells were harvested for flow cytometric analysis after incubating with medium alone or with antibodies (20 μg/mL) for 48 hours. Percentage of apoptotic cells with sub-G1 DNA content (M1 phase) was indicated in the histograms, and the percentages of cells in different phases of the cell cycle were analyzed using Modfit software. Similar results were obtained in 3 independent experiments. HeFi-1 caused cell-cycle arrest as defined by the reduced percentage of the cells in S phase and the increased percentage in G1 phase when compared with those of the cells treated with medium alone or B3 antibody. The daclizumab did not show induction of apoptosis or cell-cycle arrest.
Measurement of karpas299 lymphoma growth in SCID/NOD wild-type mice (n = 30). (A) Kaplan-Meier survival plot of SCID/NOD wild-type mice bearing the karpas299 lymphoma. (B) Changes of the body weight of the mice (lines represent individual mice). (C) Average levels of serum sIL-2Rα and β2μ of the mice. (D) Individual serum sIL-2Rα and β2μ levels immediately before death. After the intravenous infusion of 1 × 107 karpas299 cells, the mice began to die around 4 weeks, and all of the karpas299-bearing mice succumbed to the lymphoma within 2 months. The body weight decreased, with a few exceptions in animals that developed big subcutaneous tumors, and serum sIL-2Rα and β2μ concentrations increased as the disease progressed in the mice. The sIL-2Rα reached levels of 25 000 to 300 000 pg/mL, and β2μ reached levels of 1 to 10 ng/mL immediately before death.
Measurement of karpas299 lymphoma growth in SCID/NOD wild-type mice (n = 30). (A) Kaplan-Meier survival plot of SCID/NOD wild-type mice bearing the karpas299 lymphoma. (B) Changes of the body weight of the mice (lines represent individual mice). (C) Average levels of serum sIL-2Rα and β2μ of the mice. (D) Individual serum sIL-2Rα and β2μ levels immediately before death. After the intravenous infusion of 1 × 107 karpas299 cells, the mice began to die around 4 weeks, and all of the karpas299-bearing mice succumbed to the lymphoma within 2 months. The body weight decreased, with a few exceptions in animals that developed big subcutaneous tumors, and serum sIL-2Rα and β2μ concentrations increased as the disease progressed in the mice. The sIL-2Rα reached levels of 25 000 to 300 000 pg/mL, and β2μ reached levels of 1 to 10 ng/mL immediately before death.
Therapeutic study of karpas299 lymphoma-bearing SCID/NOD wild-type mice with HeFi-1 and daclizumab (n = 10). (A) Serum sIL-2Rα levels in different groups at day 21 after treatment. Data represent mean ± SD. (B) Kaplan-Meier survival plot of the mice. The animals treated with HeFi-1 at a dose of 100 μg weekly for 4 weeks had decreased serum values of sIL-2Rα (P < .01) and prolonged survival (P < .01) when compared with the mice in the control group. Daclizumab treatment also prolonged the survival of the mice as compared with that in the control group (P < .05) and reduced the serum concentration of sIL-2Rα, although the difference in serum sIL-2Rα levels between the daclizumab treatment group and the control group was not statistically significant at day 21.
Therapeutic study of karpas299 lymphoma-bearing SCID/NOD wild-type mice with HeFi-1 and daclizumab (n = 10). (A) Serum sIL-2Rα levels in different groups at day 21 after treatment. Data represent mean ± SD. (B) Kaplan-Meier survival plot of the mice. The animals treated with HeFi-1 at a dose of 100 μg weekly for 4 weeks had decreased serum values of sIL-2Rα (P < .01) and prolonged survival (P < .01) when compared with the mice in the control group. Daclizumab treatment also prolonged the survival of the mice as compared with that in the control group (P < .05) and reduced the serum concentration of sIL-2Rα, although the difference in serum sIL-2Rα levels between the daclizumab treatment group and the control group was not statistically significant at day 21.
Kaplan-Meier survival plot of karpas299 lymphoma-bearing SCID/NOD wild-type and SCID/NOD FcRγ–/– mice treated with HeFi-1 and daclizumab. (A) SCID/NOD wild-type mice (n = 15). (B) SCID/NOD FcRγ–/– mice (n = 10-12). Treatment with daclizumab prolonged the survival of karpas299 lymphoma-bearing SCID/NOD wild-type mice significantly when compared with the control group (P < .01). However, the therapeutic efficacy of daclizumab was lost in karpas299 lymphoma-bearing SCID/NOD FcRγ–/– mice. In contrast, HeFi-1 showed a similar therapeutic efficacy in SCID/NOD wild-type as compared to SCID/NOD FcRγ–/– mice bearing the karpas299 lymphoma. The lymphoma-bearing mice in HeFi-1 treatment groups had a significantly prolonged survival when compared with mice in the control groups (P < .01).
Kaplan-Meier survival plot of karpas299 lymphoma-bearing SCID/NOD wild-type and SCID/NOD FcRγ–/– mice treated with HeFi-1 and daclizumab. (A) SCID/NOD wild-type mice (n = 15). (B) SCID/NOD FcRγ–/– mice (n = 10-12). Treatment with daclizumab prolonged the survival of karpas299 lymphoma-bearing SCID/NOD wild-type mice significantly when compared with the control group (P < .01). However, the therapeutic efficacy of daclizumab was lost in karpas299 lymphoma-bearing SCID/NOD FcRγ–/– mice. In contrast, HeFi-1 showed a similar therapeutic efficacy in SCID/NOD wild-type as compared to SCID/NOD FcRγ–/– mice bearing the karpas299 lymphoma. The lymphoma-bearing mice in HeFi-1 treatment groups had a significantly prolonged survival when compared with mice in the control groups (P < .01).
Average changes of the body weight of karpas299 lymphoma-bearing SCID/NOD wild-type and SCID/NOD FcRγ–/– mice during the course of the treatment. (A) SCID/NOD wild-type mice (n = 15). (B) SCID/NOD FcRγ–/– mice (n = 10-12). The body weight of both SCID/NOD wild-type and SCID/NOD FcRγ–/– mice bearing karpas299 lymphoma in the control groups decreased rapidly after 2 weeks from the start of the experiment. HeFi-1 treatment delayed the decrease of the body weight of both SCID/NOD wild-type and SCID/NOD FcRγ–/– mice. The daclizumab treatment slowed down the decrease of body weight of SCID/NOD wild-type mice but did not show this effect in SCID/NOD FcRγ–/– mice. *P < .05, **P < .01.
Average changes of the body weight of karpas299 lymphoma-bearing SCID/NOD wild-type and SCID/NOD FcRγ–/– mice during the course of the treatment. (A) SCID/NOD wild-type mice (n = 15). (B) SCID/NOD FcRγ–/– mice (n = 10-12). The body weight of both SCID/NOD wild-type and SCID/NOD FcRγ–/– mice bearing karpas299 lymphoma in the control groups decreased rapidly after 2 weeks from the start of the experiment. HeFi-1 treatment delayed the decrease of the body weight of both SCID/NOD wild-type and SCID/NOD FcRγ–/– mice. The daclizumab treatment slowed down the decrease of body weight of SCID/NOD wild-type mice but did not show this effect in SCID/NOD FcRγ–/– mice. *P < .05, **P < .01.
Discussion
Passive immunotherapies using monoclonal antibodies have manifested great success with 19 therapeutic monoclonal antibodies approved by the Food and Drug Administration, including 8 directed toward the treatment of cancer.21-23 A diverse array of strategies and cellular targets of antibody action have been employed in patients with cancer.24-26 Cellular targets of monoclonal antibody action include the tumor cells themselves, tumor vasculature, as well as an array of host-negative immunoregulatory cellular elements (checkpoints). The mechanisms of action of antibodies directed toward the tumor cells themselves include activation of death pathways within the tumor cells, complement-mediated cell killing, antibody-mediated delivery of cytotoxic agents to the tumor cells, as well as antibody-dependent cellular cytotoxicity (ADCC).
The present study was focused on the mechanism underlying the inhibition of the tumor cell growth mediated by the anti-CD30 monoclonal antibody, HeFi-1, in a murine model of ALCL. A major focus of the study was on the requirement for the expression of the FcRγIII receptor. ADCC-mediated target cell killing requiring the expression of FcRγIII that involves the redirection of host cytotoxic mononuclear cells to the tumor plays a major role in the action of many monoclonal antibodies in murine tumor model systems.27-31 In particular, Clynes and colleagues,27 using FcRγ–/– mice that do not express FcRγIII, the stimulatory Fc receptor, demonstrated that the efficacy of an antibody directed toward malignant melanoma cells was greatly diminished in such Fc receptor–deficient mice. Similarly, they showed that FcRγIII is required for the optimal actions of trastuzumab in the treatment of an HER-2/neu–expressing breast tumor and of rituximab in the treatment of a CD20-expressing B-cell lymphoma in murine models.28 Recently, they defined another common γ chain–dependent activating Fc receptor, FcγRIV, to which murine IgG2a and IgG2b bound with intermediate affinity.32 In contrast, murine IgG1 did not bind to this receptor. Human IgG1 preferentially bound to mouse FcγRIV, in addition to binding to mouse FcγRIII, accounting for its FcγR-dependent action in vivo.32 We have demonstrated the requirement for FcγR expression in the action of the CD25-directed monoclonal antibodies daclizumab, murine anti-Tac and 7G7/B6, the CD52-directed monoclonal antibody CAMPATH-I, and the CD2-directed monoclonal antibody MEDI-507, in a murine model of human adult T-cell leukemia.29-31 In contrast, Clynes et al28 demonstrated that the disruption of the gene that encodes the inhibitory FcRIIγB-Ig binding receptor substantially enhanced antitumor activity. In contrast to these observations with other monoclonal antibodies, active therapy of the karpas299 ALCL model mediated by the anti-CD30 monoclonal antibody, HeFi-1, did not require FcRγIII expression. In particular, we demonstrated significant inhibition of tumor growth as monitored by the survival of karpas299 ALCL–bearing mice in both SCID/NOD wild-type and SCID/NOD FcRγ–/– mice. These results, taken in conjunction with our observation in vitro of a direct antiproliferative and anti–cell-cycle progression action, support the view that the anti-CD30 monoclonal antibody observed with karpas299 ALCL cells represented a direct action on the tumor cells themselves.
In previous studies as well as the present study, HeFi-1 was effective in the treatment of mice bearing the karpas299 ALCL.12 However, quite diverse responses have been noted with other anti-CD30 antibodies and with other tumor cell lines.10,12-14 Effective therapy has been limited to anti-CD30 monoclonal antibodies, such as HeFi-1, which interact with the CD30 ligand binding site10-12 and were not observed with antibodies such as Ber-H2, which interact with a nonbinding site of CD30.12 Furthermore, monoclonal antibodies, which recognize the ligand-binding site on CD30, have been ineffective in the therapy of certain other CD30-expressing tumors, especially those of Hodgkin disease.10 Since HeFi-1 action on karpas299 cells did not require the expression of FcRγIII, an alternate direct action on the tumor cells must be sought. There is considerable controversy in the literature concerning the mechanism of action of anti-CD30 antibodies, with the focus either on apoptotic cell death of ALCL cells or, alternatively, on an inhibition of cell-cycle progression. Duckett and colleagues10,33 reported that anti-CD30 treatment leads to the apoptotic cell death of karpas299 cells. By way of background, they indicated that TNF acting through TNF-R1 can either induce apoptosis through the action of Fas-associated death domain (FADD) or alternatively promote survival through TNF receptor–associated factor (TRAF) recruitment and NF-kB induction. They provide evidence indicating that CD30-directed therapy of ALCL cells led to the selective reduction of TRAF-2 and to the impairment of the ability of these cells to activate the prosurvival NF-kB. In contrast, Hodgkin disease cells, which constitutively express NF-kB in the nucleus, were not susceptible to anti-CD30–induced apoptosis but could be sensitized following inhibition of NF-kB.10 These studies suggested that NF-kB plays a determining role in the sensitivity or resistance of lymphoma cells to CD30-induced apoptosis.10
Tian and coworkers12 also indicated that antitumor effects of unconjugated HeFi-1 involved apoptosis of the target ALCL cells. However, Schneider and coworkers14 provide an alternative perspective. They reported that the activation of CD30 did not lead to the cleavage of pro–caspase-3 and that in all examined cells and cell lines, cell death was not mediated by anti-CD30. However, in the cell lines where growth was inhibited, this restrained cell growth was accompanied by the expression of the cell-cycle inhibitor p21WAF1/CIP1, and p38 MAP kinase was involved in the anti-CD30– mediated events.14 The discrepancies between studies may reflect differences in the antibodies used or in the sublines of the CD30-positive cells examined and reflect the more general pleiotropic effects of CD30 activation. Taken as a whole, the studies suggest that in select cases unmodified anti-CD30 monoclonal antibodies might provide effective therapy for patients, especially in those with CD30-expressing ALCL. Nevertheless, in most cases, especially those with Hodgkin lymphoma, such an approach would not be effective. In such cases, alternative strategies would be required to take advantage of the contrasting expressions of CD30 between an array of tumor cells where it is strongly expressed and normal tissues where expression is limited. Such CD30-directed approaches include monoclonal antibody single-chain Fv-toxin conjugates as well as anti-CD30 monoclonal antibodies armed with radionuclides.1,34-36
In conclusion, in the present study it was demonstrated that the agonist action of the anti-CD30 monoclonal antibody, HeFi-1, inhibited the growth of karpas299 cells in vitro and in vivo, unlike the anti-CD25 monoclonal antibody, daclizumab, which inhibited the growth of karpas299 cells only in vivo. Furthermore, we demonstrated the therapeutic efficacy of daclizumab only in the karpas299-bearing SCID/NOD wild-type mice and not in the karpas299-bearing SCID/NOD FcRγ–/– mice, results comparable to those of the antibody in the MET-I (an adult T-cell leukemia) model system.29 However, we demonstrated the therapeutic efficacy of HeFi-1 in the ALCL model in both SCID/NOD wild-type and SCID/NOD FcRγ–/– mice, suggesting that in contrast to an array of other monoclonal antibodies in murine tumor model systems,27-31 the expression of the receptor FcRγIII was not required for the effective action of HeFi-1 in this mouse ALCL model.
Prepublished online as Blood First Edition Paper, March 21, 2006; DOI 10.1182/blood-2005-11-4607.
Supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
M.Z. designed and performed the research, analyzed the data, and wrote the paper; Z.Y. performed the research, and reviewed and revised the paper; Z.Z. performed the research; K.G. performed the research; C.K.G. contributed to design of the research and reviewed and revised the paper; J.V.R. provided SCID/NOD FcRγ–/– mice and reviewed and revised the paper; J.J. contributed to the design of the research and reviewed and revised the paper; M.W.B. contributed to design of the research and reviewed and revised the paper; and T.A.W. designed the research and wrote the paper.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Ira Pastan for providing the B3 antibody.

![Figure 2. The effect of HeFi-1 and daclizumab on the proliferation and cell cycle of karpas299 cells in vitro. (A) Karpas299 cells were incubated with medium alone or with antibodies (20 μg/mL) at 37°C for 42 hours and pulsed with 1 μCi (0.037 MBq) [3H]thymidine for 6 hours. The cells then were harvested and counted. The data represent mean ± SD of triplicates and are representative of 3 experiments. HeFi-1 inhibited the proliferation of karpas299 cells, whereas daclizumab did not inhibit the proliferation. CPM indicates counts per minute. (B) The cells were harvested for flow cytometric analysis after incubating with medium alone or with antibodies (20 μg/mL) for 48 hours. Percentage of apoptotic cells with sub-G1 DNA content (M1 phase) was indicated in the histograms, and the percentages of cells in different phases of the cell cycle were analyzed using Modfit software. Similar results were obtained in 3 independent experiments. HeFi-1 caused cell-cycle arrest as defined by the reduced percentage of the cells in S phase and the increased percentage in G1 phase when compared with those of the cells treated with medium alone or B3 antibody. The daclizumab did not show induction of apoptosis or cell-cycle arrest.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/2/10.1182_blood-2005-11-4607/2/m_zh80140698740002.jpeg?Expires=1769348737&Signature=CThqVniuAA8mbLDYofcLmk8PNix8tt5-IZDTP2Gye~5VIllw3~lPGZUOOVObplHlcZ7xd-T-2bgCOs7F3QTVhYQhmwReiGLESEZar49lcvyq0SV6SMs2X1k-oTvPw71MkUcirlrQjj0jBaoFasmGzrU4IYybbnRVzEqhPmKTyDp1uId7aDWTYQU0z2NF3LEcDQBdq3oNFYRWDoe3CiV~SNHIPAaA3i3VwhW0ZwofULNOycKtyeEiIoCULY1xKcPmJNVBREcMxAAH0Ys0Op1zOGg0UevpAW~npnlENeEgLT3QLUOA9OQASf58grBdgwPgF0ynw8BiGKdzg9nf6tNAHQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)