Abstract
Adult T-cell leukemia (ATL) consists of an overabundance of T cells, which express CD25. Therapeutic efficacy of astatine-211 (211At)–labeled murine monoclonal antibody 7G7/B6 alone and in combination with daclizumab was evaluated in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice given injections of MET-1 human T-cell leukemia cells. Daclizumab and 7G7/B6 are directed toward different epitopes of CD25. Either a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6 per mouse given intravenously or receptor-saturating doses of daclizumab given at 100 μg weekly for 4 weeks intravenously inhibited tumor growth as monitored by serum levels of human β-2 microglobulin (β2μ) and by prolonged survival of leukemia-bearing mice compared with the control groups (P < .001). The combination of 2 agents enhanced the antitumor effect when compared with groups treated with 12 μCi (0.444 MBq) of 211At-7G7/B6 (P < .05) or daclizumab alone (P < .05). The median survival duration of the PBS group was 62.6 days and 61.5 days in the radiolabeled nonspecific antibody 211At-11F11–treated group. In contrast, 91% of mice in the combination group survived through day 94. These results that demonstrate a significantly improved therapeutic efficacy by combining 211At-7G7/B6 with daclizumab support a clinical trial of this regimen in patients with ATL.
Introduction
Adult T-cell leukemia (ATL) develops in a small portion of individuals infected with human T-cell lymphotrophic virus-1 (HTLV-I) and consists of an overabundance of malignant activated T cells, which are characterized by expression of the α subunit of the interleukin-2 receptor (IL-2Rα; CD25) on their cell surfaces.1-4 Presently, there is no accepted curative therapy for ATL and patients progress to death with a median survival duration of 9 months for those with acute ATL and 24 months for those with chronic ATL.1 The observation that IL-2Rα is not expressed by normal resting cells but is expressed by ATL cells provided the rationale for the use of monoclonal antibodies (mAbs) directed toward IL-2Rα to deliver therapeutic agents.5 Some partial and rare complete remissions were obtained in patients with ATL treated in clinical trials with the intact murine anti-Tac, humanized anti-Tac (daclizumab), as well as these intact antibodies armed with yttrium-90 (90Y) used in an effort to develop yet more effective IL-2Rα–directed agents.6,7 A preclinical in vivo murine model of ATL was developed by introducing leukemic cells (MET-1) from a patient with ATL into nonobese diabetic/severe combined immuno-deficient (NOD/SCID) mice, and new therapeutic approaches have been tested in this model before initiating human clinical trials.8-14 In initial studies, antibodies to IL-2Rα including daclizumab, murine anti-Tac, and 7G7/B6 inhibited the progression of the leukemia and prolonged survival of the leukemia-bearing mice. However, in general, cures were not achieved.8 Therefore, more effective therapeutic approaches were required. In previous therapeutic trials, daclizumab was combined with pretargeted radioimmunotherapy in the ATL model and achieved the complementary actions of receptor-saturating doses of daclizumab to yield antibody-dependent cellular cytotoxicity (ADCC) and cytokine deprivation–mediated leukemic cell death along with the tumor cytoreduction provided by the radiation delivered to leukemic cell surfaces.9,10 We also demonstrated that combining daclizumab with flavopiridol or with PS341 significantly improved therapeutic efficacy in the ATL model.13,14 These observations suggest that for cancer therapy, the addition of 2 therapeutic agents that function via different mechanisms of action may be greater than additive in their cytotoxic action, leading to malignant cell death.
In the clinical and preclinical trials, remissions have been observed using either the unmodified daclizumab monoclonal antibody at receptor-saturating doses or this antibody labeled at high specific activity with 90Y. However, the complementary actions of receptor-saturating doses of the daclizumab monoclonal antibody to yield ADCC and IL-2 deprivation–mediated apoptotic leukemic cell death with this radiolabeled monoclonal antibody were difficult to obtain in conjunction with the tumor cytoreduction provided by the irradiation mediated by radionuclides such as 90Y or 211At delivered by daclizumab to the leukemic cell surfaces. To obtain cytokine deprivation–mediated cell death, one must use large receptor-saturating quantities of the monoclonal antibody. However, the administration of such large quantities of monoclonal antibody armed with a radionuclide leads to low specific activity and a decreased proportion of administered radiolabeled antibody delivered and bound to tumor cells. The resulting circulating unbound radiolabeled antibody yields unacceptable bone marrow toxicity which in turn reduces the maximum dose of radioactivity that can be administered. In this study, we address the limitations inherent in the use of a single radiolabeled monoclonal antibody to simultaneously saturate receptors and deliver a high proportion of the administered radionuclide to the tumor cells by simultaneously using 2 non–cross-competing antibodies, daclizumab and 7G7/B6, that bind to different epitopes of the IL-2Rα protein on the surfaces of leukemia/lymphoma cells. We used daclizumab at a receptor-saturating dose in combination with small quantities of 7G7/B6 armed at high specific activity with a radionuclide.
Monoclonal antibodies (mAbs) directed against tumor-associated antigens armed with diverse radionuclides are being investigated as therapeutic agents for the treatment of malignant disease.15-17 Although encouraging results have been obtained in the treatment of lymphoma and other diseases with mAbs radiolabeled with β–-emitting radionuclides, further development is needed before an ideal radioimmunotherapeutic agent is achieved.7,18 The α-emitting radionuclides appear to have several advantages when compared with β–-emitting radionuclides in radioimmunotherapy, especially with isolated malignant cells as in leukemia. The high linear energy transfer of α particles makes them highly cytotoxic with a relative biologic effectiveness 5 to 20 times that of β particles.19-21 Another advantage of α particles compared with β– particles is that they exhibit a low dependence on dose rate and oxygen enhancement effects. In addition, α particles have relatively short effective path lengths in tissue that decrease the radiation delivered to normal tissues.19,22,23 Among the α emitters, currently under investigation for use in radioimmunotherapy, 211At is perhaps the most promising candidate for radioimmunotherapeutic applications on the basis of half-life considerations (t1/2 = 7.2 h).
In this study, the therapeutic efficacy of 211At-labeled 7G7/B6 was investigated in a murine model of ATL alone and in combination therapy with daclizumab. The scientific hypothesis supporting the use of the combination is that the 2 therapeutic agents employ different mechanisms of action and might manifest additive or synergistic efficacy. Both antibodies are directed toward the same target, IL-2Rα, but bind different epitopes on that target. In the present study, we obtained very promising results with this combination regimen for the treatment of IL-2Rα–expressing T-cell malignancy.
Materials and methods
Monoclonal antibody
7G7/B6 is a mouse IgG2a mAb directed toward an epitope of the IL-2Rα peptide distinct from that identified by daclizumab, which recognizes IL-2Rα.24 It was reported that a murine IgG2a such as 7G7/B6 has the feature of nonspecific binding to spleen and liver that can be blocked by the administration of another murine IgG2a–irrelevant antibody.25 In this study, UPC10, a murine IgG2a monoclonal antibody that does not recognize resting or activated peripheral blood mononuclear cells or cell lines including T cell, B cell, and monocyte populations, was used as an agent that blocks the nonspecific binding of radiolabeled 7G7/B6 in the spleen and liver of nude and NOD/SCID mice. The plasmacytoma producing UPC10 was obtained from Michael Potter at the National Cancer Institute (NCI; Bethesda, MD). The control monoclonal antibody 11F11 is a mouse IgG2a mAb that recognizes the Shiga-like toxin II of enterohemorrhagic Escherichia. The hybridoma producing 11F11 was obtained from Alison D. O'Brien (Department of Microbiology, Uniformed Services University of Health Science, Bethesda, MD).26
Radiolabeling of monoclonal antibodies
Production and purification of 211At as well as the procedure for 211At labeling of 7G7/B6 and 11F11 were recently reported in detail.27,28 In brief, 211At was produced employing the 209Bi (α2n) 211At reaction by irradiating an external or internal bismuth target with an α beam from a Cyclotron Corporation CS-30 cyclotron (Berkeley, CA). The 211At was isolated as described previously.27 The 211At was linked to 7G7/B6 and 11F11 as described previously using N-succinimidyl N-(4-{211At}astatophenethyl) succinamate (SAPS).28 Specific activities obtained for the isolated products were 2.7 and 4.6 μCi/μg (0.0999 and 0.1702 MBq/μg), respectively.
Binding integrity of radiolabeled antibody
Radiolabeling of 7G7/B6 antibody could theoretically alter its capacity to bind to the IL-2R receptor. Radiolabeled preparations were tested to determine the proportion of the radiolabeled product that bound to the IL-2Rα–expressing human leukemic T-cell line Kit-225-IG3. For each preparation, triplicates of 3 × 105, 1 × 106, and 3 × 106 Kit-225-IG3 cells were placed in PBS buffer with 10% FCS, 0.2% NaN3 in screw-cap vials. Fifty-microliter aliquots of the radiolabeled (211At) mAbs were added separately to each vial. After incubation at 4°C for 20 minutes, the vials were centrifuged for 4 minutes (setting 10) on an Eppendorf (Westbury, NY) centrifuge. The supernatant was aspirated and the radioactivity in the pellets was determined compared with a standard. Nonspecific radioactivity was quantitated similarly with the exception that a 100-fold molar excess of unmodified 7G7/B6 was added to each tube. The nonspecific counts were less than 3% of the added counts in all cases. The bind ability was measured as follows: (pellet counts – nonspecific counts)/(initial sample counts).
Mouse model of ATL
The ATL cell population, MET-1, was established from the peripheral blood of a patient with acute ATL and the cells were maintained by serial transfer in NOD/SCID mice (Jackson Laboratories, Bar Harbor, ME). MET-1 cells have a distinct phenotype elucidated by fluorescence-activated cell sorting (FACS) analysis: CD3dim, CD4+/–, CD7–, CD20–, and CD25+. The leukemia model was established by the intraperitoneal injection of 1.5 × 107 MET-1 cells into NOD/SCID mice as described previously.8 Intraperitoneal injection of 1.5 × 107 MET-1 cells in NOD/SCID mice results in a wide tissue distribution of leukemia cells with marked infiltration of the spleen, liver, lymph nodes, lung, kidney, and moderate infiltration of the bone marrow. Therapeutic trials were performed on these mice when their serum-soluble IL-2Rα (sIL-2Rα) levels were from 1000 to 10 000 pg/mL approximately 10 to 14 days after inoculation.
Monitoring of tumor growth
Throughout the therapeutic trials, the serum concentrations of soluble human sIL-2α and human β2-microglobulin (β2μ) that were used as surrogate tumor markers were measured using enzyme-linked immunosorbent assay (ELISA) kits purchased from R&D Systems (Minneapolis, MN). The ELISAs were performed as suggested in the manufacturer's kit inserts.
Definition of the maximum tolerated dose
Prior to initiation of therapeutic studies, the maximum tolerated dose of 211At-7G7/B6 was determined in non–tumor-bearing NOD/SCID mice. Single doses of 6, 12, 24, 40, and 70 μCi (0.222, 0.444, 0.888, 1.48, and 2.59 MBq) of 211At-7G7/B6 per mouse were administered intravenously 30 minutes after 400 μg of UPC10 blocking antibody given intravenously. During this experiment, body weight, platelets, and white blood cells were monitored. Deaths were observed in a proportion of mice receiving doses of higher than 12 μCi (0.444 MBq). Therefore, a dose of 12 μCi (0.444 MBq) of 211At-7G7/B6 was used in the therapeutic trials.
Two mice from each group (normal control and groups receiving 6, 12, 24, and 40 μCi [0.222, 0.444, 0.888, 1.48 MBq; surviving mice] of 211At-7G7/B6) had a histologic examination 5 months after 211At-7G7/B6 was given. Typically, pathologic evaluations of liver, kidney, intestine, lung, bone marrow, and heart were performed. Tissue samples were harvested and fixed in 10% formalin for pathologic studies. The tissues were examined by a veterinary pathologist.
Therapeutic protocol
Therapeutic trials were performed in MET-1 leukemia-bearing mice with a tumor burden corresponding to sIL-2Rα values of 1000 to 10 000 pg/mL. The blocking antibody UPC10 (400 μg/mouse) was given intravenously before 211At-7G7/B6 or 211At-11F11 were administered. There were 5 groups in the therapeutic trials. Group 1 received a dose of 12 μCi (0.444 MBq) of 211At-7G7/B6 (specific activity 4.6 μCi/μg [0.1702 MBq/μg] and 10 μg/mouse of 7G7/B6 antibody). Group 2, the immunotherapy (daclizumab) group, was given injections of 100 μg of daclizumab on days 0, 7, 14, and 21. Group 3, the combination therapy group, received a combined therapy of 12 μCi (0.444 MBq) of 211At-7G7/B6 on day 0 with daclizumab given on days 0, 7, 14, and 21. Group 4 received 12 μCi (0.444 MBq) of 211At-11F11 (specific activity 2.7 μCi/μg [0.0999 MBq/μg] and 10 μg/mouse of 11F11 antibody) as 211At-labeled nonspecific mAb control. Group 5 received 200 μL PBS weekly for 4 weeks and served as a control for the daclizumab immunotherapy. The groups were randomly assigned and had comparable average serum concentrations of the surrogate tumor marker, sIL-2Rα (mean ∼ 2600 pg/mL), at the beginning of the experiments.
Statistics analysis
The serum levels of β2μ were analyzed at different time points for the different treatment groups using the Student t test for unpaired data. Statistical significance of differences in survival of mice in different groups was determined by the log-rank test using the StatView program (Abacus Concepts, Berkeley, CA)
Results
Binding integrity of radiolabeled antibody
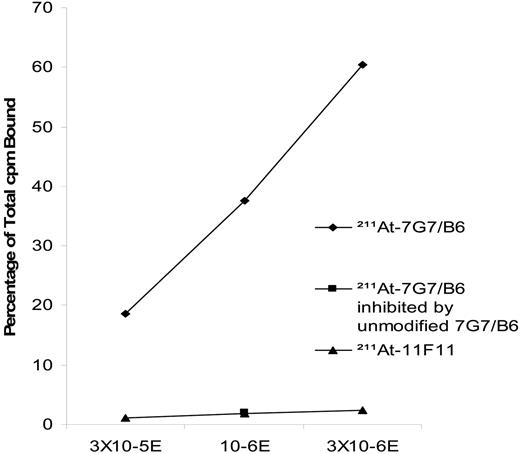
A competitive binding assay was performed following each labeling procedure. In these studies, the binding of 211At-labeled 7G7/B6 antibody to IL-2Rα was specifically inhibited by unmodified 7G7/B6 at increasing concentrations (100 molar excess) using a competition assay wherein inhibition of binding of nonsaturating amounts of 211At-7G7/B6 antibody was assessed using the IL-2Rα–expressing Kit-225-IG3 cell (Figure 1). The 211At-11F11 control antibody did not specifically bind to IL-2Rα–expressing Kit-225-IG3 cell.
Definition of the maximum tolerated dose and toxicity
Different doses of 211At-7G7/B6 (6, 12, 24, 40, and 70 μCi [0.222, 0.444, 0.888, 1.48, and 2.59 MBq]) were administered to the mice intravenously. The survival of mice in each group was monitored. All mice died within 10 days in the group receiving 70 μCi (2.59 MBq) of 211At-7G7/B6. Two of 5 mice died within 14 days and 1 mouse died on day 33 in the group receiving 40 μCi (1.48 MBq) of 211At-7G7/B6. One mouse died on day 85 and another died on day 108 in the 24 μCi (0.888 MBq) 211At-7G7/B6 group. There were no deaths in the groups receiving 6 and 12 μCi (0.222 and 0.444 MBq) of 211At-7G7/B6 during the 5-month period of observation.
The binding of 211At-7G7/B6 to IL-2Rα on Kit-225-IG3 cells was specifically inhibited by unmodified 7G7/B6 mAb. A competitive binding assay was performed as described in “Binding integrity of radiolabeled antibody” under “Materials and methods.” The line with diamonds shows the binding of 211At-7G7/B6 mAb to IL-2Rα–expressing Kit-225-IG3 cells at 3 × 105, 1 × 106, and 3 × 106 cells. The point with the square symbol is the 211At-7G7/B6 mAb binding to IL-2Rα when blocked by the addition of a 100-fold greater concentration of unmodified 7G7/B6. The line with triangles represents 211At-11F11 mAb binding to the IL-2Rα–expressing Kit-225-IG3 cell. cpm indicates counts per minute.
The binding of 211At-7G7/B6 to IL-2Rα on Kit-225-IG3 cells was specifically inhibited by unmodified 7G7/B6 mAb. A competitive binding assay was performed as described in “Binding integrity of radiolabeled antibody” under “Materials and methods.” The line with diamonds shows the binding of 211At-7G7/B6 mAb to IL-2Rα–expressing Kit-225-IG3 cells at 3 × 105, 1 × 106, and 3 × 106 cells. The point with the square symbol is the 211At-7G7/B6 mAb binding to IL-2Rα when blocked by the addition of a 100-fold greater concentration of unmodified 7G7/B6. The line with triangles represents 211At-11F11 mAb binding to the IL-2Rα–expressing Kit-225-IG3 cell. cpm indicates counts per minute.
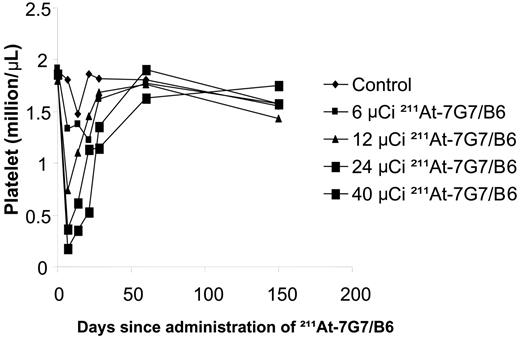
Platelet counts were reduced in the mice receiving 211At-7G7/B6 in a dose-related manner (Figure 2). The nadir occurred 1 week after radiation therapy and recovered 2 to 3 weeks later. The white blood cell (WBC) count followed the same pattern as the platelet counts in the 211At-7G7/B6–treated mice. The WBC count was reduced with 211At-7G7/B6 in a dose-related manner. The nadir occurred 1 week after radiation therapy and recovered 2 to 3 weeks later. The mean of WBC counts at 1 week after 211At-7G7/B6 was given were 3.769 × 109/L (3769/μL; control), 1.963 × 109/L (1963/μL; 6 μCi 211At-7G7/B6), 1.85 × 109/L (1850/μL; 12 μCi 211At-7G7/B6), 1.123 × 109/L (1123/μL; 24 μCi 211At-7G7/B6), and 0.71 × 109/L (710/μL; 40 μCi 211At-7G7/B6 in surviving mice). Slight body weight loss (3%) was observed in the mice that received 12 μCi (0.444 MBq) of 211At-7G7/B6 from the second to the fourth week but the animals recovered subsequently, whereas mice that received 24 μCi (0.888 MBq) of 211At-7G7/B6 showed a significant loss of body weight (9%; P < .05) within 1 week after the 211At-7G7/B6 was given but those animals also recovered 2 to 3 weeks later. There was no body weight loss in the life span control and 6 μCi (0.222 MBq) 211At-7G7/B6 groups.
There were no abnormal pathologic findings at 5 months in 211At-7G7/B6–treated mice compared with normal mice.
Effective treatment of ATL using 211At-7G7/B6 combined with daclizumab directed toward CD25
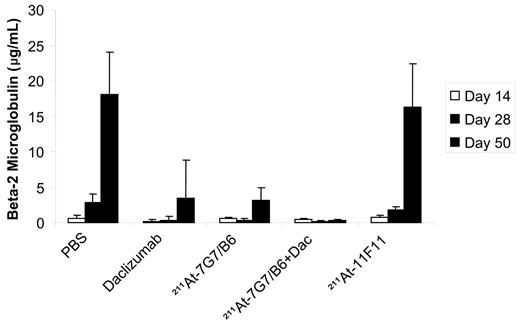
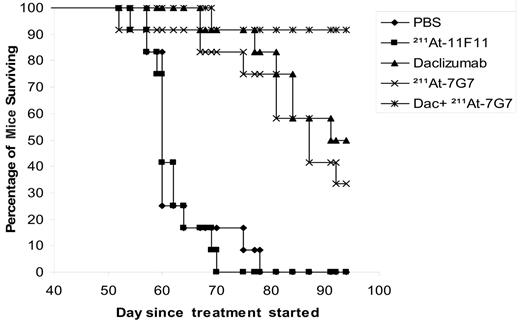
In a therapeutic trial in the MET-1 model of human ATL, a dose of 12 μCi (0.444 MBq) of 211At-7G7/B6 (10 μg), daclizumab (100 μg intravenously/wk × 4), and the combination of 12 μCi (0.444 MBq) of a single dose of 211At-7G7/B6 with daclizumab (100 μg intravenously/wk × 4) demonstrated therapeutic efficacy as indicated by their effect on the serum levels of human β2μ, a surrogate tumor marker (Figure 3), and on the survival of ATL-bearing mice (Figure 4). When compared with the serum concentrations of human β2μ in the PBS and 211At-11F11 nonspecific monoclonal antibody control groups of mice, on days 28 and 50 there were significant reductions of serum β2μ levels in the groups of mice receiving 12 μCi (0.444 MBq) of 211At-7G7/B6 (P < .001), 4-week daclizumab (P < .001), and the combination of 12 μCi (0.444 MBq) of 211At-7G7/B6 and 4-week daclizumab (P < .001). The human β2μ level decreased progressively in the combination group during the treatment period and even after the treatment was completed as shown in Figure 3, whereas the human β2μ level increased after treatment was completed in the daclizumab alone group and in the group receiving a single dose of 211At-7G7/B6. Furthermore, there were significant prolongations of the survival of the groups of mice treated with 211At-7G7/B6 (P < .001), 4-week daclizumab (P < .001), and the combination of 211At-7G7/B6 with daclizumab (P < .001) when compared with PBS or 211At-11F11 groups (Figure 4). In addition, there was a significant prolongation of the survival of the mice that were treated with the combination of 211At-7G7/B6 with daclizumab when compared with a single dose of 211At-7G7/B6 or with 4-week daclizumab when administered independently (P < .05). The median survival duration of the control group (PBS) was 62.6 days and 61.5 days in the 211At-11F11–treated group. All mice in PBS and 211At-11F11 groups died by day 78 of the study. In contrast, there were 36% of mice in the 211At-7G7/B6 group, 50% of mice in the daclizumab-alone group, and 91% of mice in the combination group surviving on day 94. Comparable efficacy of 211At-7G7/B6 compared with control group in the therapy of ATL was observed when the study was repeated in an additional experiment.
Platelet counts were measured in NOD/SCID mice that received different quantities of 211At. The circulating platelet counts were determined initially at weekly intervals and subsequently at monthly intervals. Mice in the control group did not receive any treatment (control). Mice in the other groups had received 400 μg of UPC10 mAb intravenously 30 minutes before they received 211At-7G7 at doses of 6, 12, 24, and 40 μCi (0.222, 0.444, 0.888, and 1.48 MBq).
Platelet counts were measured in NOD/SCID mice that received different quantities of 211At. The circulating platelet counts were determined initially at weekly intervals and subsequently at monthly intervals. Mice in the control group did not receive any treatment (control). Mice in the other groups had received 400 μg of UPC10 mAb intravenously 30 minutes before they received 211At-7G7 at doses of 6, 12, 24, and 40 μCi (0.222, 0.444, 0.888, and 1.48 MBq).
Inhibition of the growth of MET-1 ATL cells in NOD/SCID mice by 211At-7G7/B6, daclizumab, and the combination of these agents. MET-1 ATL cells were transferred into mice intraperitoneally. The groups (11-12 mice/group) included those receiving PBS, 4 weekly doses of 4 mg/kg (100 μg/mouse) daclizumab (Dac), a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6 (10 μg/mouse), the combination 100 μg of 4-weekly doses of daclizumab with a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6, and a single dose of 12 μCi (0.444 MBq) 211At-11F11 (10 μg/mouse). The data represent the mean serum concentrations of the surrogate tumor marker human β2μ in μg/mL. The group receiving a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6, the group receiving 4-week daclizumab, and the group receiving the combination of 12 μCi (0.444 MBq) 211At-7G7/B6 with 4-week daclizumab had significantly decreased values of β2μ (0.24 μg/mL at day 50) when compared with those of the PBS and 211At-11F11 control groups (P < .001). There was no significant difference in β2μ levels between the group receiving a single dose of 12 μCi (0.444 MBq) 211At-11F11 and that receiving PBS (P = .43).
Inhibition of the growth of MET-1 ATL cells in NOD/SCID mice by 211At-7G7/B6, daclizumab, and the combination of these agents. MET-1 ATL cells were transferred into mice intraperitoneally. The groups (11-12 mice/group) included those receiving PBS, 4 weekly doses of 4 mg/kg (100 μg/mouse) daclizumab (Dac), a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6 (10 μg/mouse), the combination 100 μg of 4-weekly doses of daclizumab with a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6, and a single dose of 12 μCi (0.444 MBq) 211At-11F11 (10 μg/mouse). The data represent the mean serum concentrations of the surrogate tumor marker human β2μ in μg/mL. The group receiving a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6, the group receiving 4-week daclizumab, and the group receiving the combination of 12 μCi (0.444 MBq) 211At-7G7/B6 with 4-week daclizumab had significantly decreased values of β2μ (0.24 μg/mL at day 50) when compared with those of the PBS and 211At-11F11 control groups (P < .001). There was no significant difference in β2μ levels between the group receiving a single dose of 12 μCi (0.444 MBq) 211At-11F11 and that receiving PBS (P = .43).
Kaplan-Meier survival plot of MET-1–bearing NOD/SCID mice. The groups (11-12 mice/group) included those receiving intravenous PBS, 4 weekly doses of 4 mg/kg (100 μg/mouse) daclizumab, a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6, a single dose of 12 μCi (0.444 MBq) 211At-11F11, and the combination of 4 mg/kg of 4-week daclizumab and a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6. Survival in the treated groups was followed out to 94 days. The group receiving 4-week daclizumab, the group receiving a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6, and the group receiving a 4-week combination of 4 mg/kg daclizumab with a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6 had significantly prolonged survivals when compared with the PBS group or with the control group receiving the single dose of 12 μCi (0.444 MBq) 211At-11F11 (P < .001). The combination treatment of tumor-bearing mice with a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6 and daclizumab for 4 weeks significantly prolonged the survival of the group when compared with the group that received 4-week daclizumab or the group that received a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6 (P < .05).
Kaplan-Meier survival plot of MET-1–bearing NOD/SCID mice. The groups (11-12 mice/group) included those receiving intravenous PBS, 4 weekly doses of 4 mg/kg (100 μg/mouse) daclizumab, a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6, a single dose of 12 μCi (0.444 MBq) 211At-11F11, and the combination of 4 mg/kg of 4-week daclizumab and a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6. Survival in the treated groups was followed out to 94 days. The group receiving 4-week daclizumab, the group receiving a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6, and the group receiving a 4-week combination of 4 mg/kg daclizumab with a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6 had significantly prolonged survivals when compared with the PBS group or with the control group receiving the single dose of 12 μCi (0.444 MBq) 211At-11F11 (P < .001). The combination treatment of tumor-bearing mice with a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6 and daclizumab for 4 weeks significantly prolonged the survival of the group when compared with the group that received 4-week daclizumab or the group that received a single dose of 12 μCi (0.444 MBq) 211At-7G7/B6 (P < .05).
Discussion
ATL is a malignancy of T lymphocytes with a median survival duration of 9 months in the acute form of the disease. Various combination chemotherapies have not significantly increased the survival of patients with ATL.1 In light of the disappointing results using conventional combination chemotherapy, therapies were developed that use unmodified murine and humanized antibodies (eg, anti-Tac, daclizumab) directed toward IL-2Rα. Although such therapy yielded partial or complete remissions in one third of patients, most patients subsequently suffered a disease relapse.6,7 The use of monoclonal antibodies armed with toxins or radionuclides specifically targeting these cytotoxic agents to leukemic cells provided a valuable augmentation of therapy.7 There are a number of components that must be considered in designing an optimal radioimmunotherapy regimen, including (A) the selection of the antigenic target and thus the monoclonal antibody; (B) the choice of the linking agent to join the radionuclide to the monoclonal antibody; and (C) the choice of the radionuclide used.
As just noted, a pivotal issue to be addressed is the selection of the monoclonal antibody that targets the tumor and thereby the type of malignancy chosen as the target for radioimmunotherapy. In the present study, we have chosen different epitopes of the human IL-2Rα subunit identified by 7G7/B6 and daclizumab as our targets for immunotherapy. The scientific basis for this choice is that virtually no normal resting cells express the α subunit of the IL-2R, whereas this receptor is expressed by a high proportion of the abnormal cells in certain T-cell and B-cell lymphoid neoplasias and monocytic and granulocytic leukemias identified by anti-Tac.6 In our clinical trials, we exploit the differential expression of IL-2Rα between normal resting cells and malignant T cells. A series of modifications in the anti-Tac monoclonal antibody have been made to increase its effector function, to reduce its immunogenicity, and to improve its pharmacokinetics. To augment its potency, anti-ATL has been armed with the β–-emitting radionuclide 90Y. While a proportion of the 19 patients with HTLV-I–associated Tac underwent a remission when treated with intact anti-Tac armed with this radionuclide and were provided meaningful therapy for this form of leukemia that was previously universally fatal, only 2 of 16 patients manifested a complete remission.
A second component of an optimal radioimmunotherapeutic regimen is the choice of the chelating agent or linker to couple the radionuclide to monoclonal antibody. An ideal agent should not alter the specificity of binding of the monoclonal antibody to its antigenic target nor should it damage the antibody and thus alter its rate of catabolism or patterns of tissue distribution. The radionuclide should also be sequestered tightly so that there is no premature loss of the radionuclide from the monoclonal antibody linker complex in vivo. The agent SAPS used in the present study for 211At fulfills these requirements.27,28
A third pivotal issue in defining an optimal radioimmunotherapeutic agent is to consider the nature of the radionuclide used. A short distance of action is desirable, thus maintaining the specificity of the monoclonal antibody. Nuclear chemistry has provided a selection of α- and β–-emitting radionuclides that have a relatively short distance of action.29 A β–-emitting radionuclide such as 90Y that acts through crossfire may be preferable for the treatment of large tumor masses. In a clinical situation, an agent may eliminate nontargeted non–antigen-bearing tumor cells through the crossfire effect emanating from neighboring antigen-bearing cells that have been targeted by the radiolabeled monoclonal antibody. Nevertheless, the use of β–-emitting radionuclides has a limitation: as the target mass decreases, the benefit of the crossfire effect also decreases whereas the potential for normal tissue damage increases. With small tumors including micrometastases, individual tumor cells, and leukemic cells the therapeutic efficacy may be limited. This is because high-energy β–-emitting radionuclides deliver a high dose of radiation to normal tissues due to the longer range of β– particles. For such cellular populations, the future development of isotopic monoclonal antibody–mediated approaches may focus on α-emitting radionuclides, which may be the most effective agents at killing isolated leukemic cells without damaging normal tissues. Radionuclides emitting α particles have a high linear energy transfer (LET; 6-9 megaelectron volts [MeV] particles that act over 10 to 80 μm) and are effective at killing individual target cells.30 Thus for agents that target the surface of isolated leukemic cells, one would require the binding of only a relatively small number of radiolabeled molecules per cell to provide the limited number of nuclear transversals required for leukemic cell killing. The α-emitting radionuclides under investigation for use in systemic radioimmunotherapy include 212Bi, 213Bi, 211At, and 225Ac. A major limitation in the use of bismuth radionuclides is their short physical half-lives of 60 minutes for 212Bi and 46.6 minutes for 213Bi that impair their ability to be delivered to the target leukemic cells during their effective period of decay. Our prior study showed that 211At-labeled daclizumab significantly prolonged the mean survival time in a cynomolgus cardiac allograft model.31 This present study also focuses on 211At whose half-life of 7.2 hours may be adequate to obtain effective leukemic cell targeting. This radionuclide is of special value for antibodies such as 7G7/B6 that have been shown to stay on the lymphocyte cell surface and to not be internalized by the target cell, a process that might lead to loss of the 211At from its target arena.
In previous studies, we presented a pretargeting strategy with an anti-Tac antibody-streptavidin (HAT-SA) conjugate, which recognizes CD25, followed by 213Bi-DOTA-biotin on MET-1 and 90Y-DOTA-biotin with the SUDHL-1 murine models.9,10 The pretargeting strategy was indeed excellent especially in association with the β-emitting radionuclide 90Y with lymphoma. Since it has not been possible to use the α emitter 211At with this system, the approach was much less useful in clinical situations where there were isolated malignant cells as in leukemia or micrometastases where the crossfire effects of 90Y were less effective. Therefore we are exploring an alternative strategy that permits the use of a relatively long-lived α-emitting radionuclide and that allows the simultaneous antitumor actions provided by saturating doses of the anti-Tac (daclizumab) and the antitumor radiation delivered by 211At-7G7/B6.
In this study, an additional issue involved in systemic radioimmunotherapy was considered. We addressed the limitation inherent in the use of a single monoclonal antibody to simultaneously saturate receptors for ADCC and cytokine deprivation while delivering a high proportion of an administered radionuclide, 211At, to tumor cells by using 2 non–cross-competing antibodies, daclizumab and 7G7/B6, that bind to different epitopes of the IL-2Rα protein. Daclizumab, a mAb that binds to the IL-2–binding site of the IL-2Rα, was used at receptor-saturating doses, whereas radiolabeled 211At-7G7/B6 monoclonal antibody at high specific activity was used to provide maximum delivery of tumor irradiation. The results of a trial of a single dose of 211At-7G7/B6 in the MET-1 model of ATL were encouraging. Unmodified monoclonal antibody 7G7/B6 used at 100 μg weekly for 4 weeks showed antitumor activity in the MET-1 model in the previous studies; however, a single dose of 100 μg did not give a meaningful effect.8 The protein dose of 7G7/B6 used in this 211At-7G7/B6 trial was 10 μg so the inclusion of 211At made a major contribution to the leukemic cell killing. However, this aggressive T-cell leukemia was not completely eliminated by a single course of therapy with 211At-7G7/B6. A paradigm has emerged suggesting that increased efficacy of cancer therapy can be obtained through the addition of the additive effects of 2 agents with distinct modes of cytotoxic action leading to malignant cell death. This paradigm has been shown for the daclizumab monoclonal antibody added at therapeutic doses when labeled with 213Bi, daclizumab with flavopiridol, and the combination of trastuzumab with paclitaxel.14,32 In the present trial, we achieved the complementary action of receptor-saturating doses of the daclizumab monoclonal antibody to yield antibody-dependent cellular cytotoxicity (ADCC) and cytokine deprivation–mediated leukemic cell death along with tumor cytoreduction provided by the irradiation mediated by the radionuclide 211At delivered to the leukemic cell surfaces. Indeed, whereas neither daclizumab nor 211At-7G7/B6 alone was completely effective, remissions were observed in the majority of mice receiving both agents in conjunction (Figure 4). In conclusion, a single dose of 211At-7G7/B6 provided some efficacy, and combination therapy of 211At-7G7/B6 with repeated receptor-saturating doses of daclizumab provided the desired efficacy in the murine model of ATL investigated. Therefore, the results of the present study support the use of this combination regimen in a clinical trial involving the treatment of patients with ATL.
Prepublished online as Blood First Edition Paper, March 28, 2006; DOI 10.1182/blood-2005-11-4757.
Supported by the Intramural Program of the NIH, National Cancer Institute, Center for Cancer Research.
Z.Z. designed and conducted experiments, analyzed the data, and wrote the manuscript; M.Z. designed and performed experiments; K.G. labeled 7G7/B6 antibody with astatine; V.S.T. synthesized the astatine linker; P.S.P. produced astatine for the 7G7 labeling; B.B. performed experiments; C.G. contributed to the design of research and reviewed the manuscript; M.W.B. designed the astatine linker and critically reviewed the manuscript revision; and T.A.W. designed experiments and wrote the manuscript.
Z.Z. and M.Z. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We gratefully acknowledge Dr Alison D. O'Brien for providing the hybridoma that produces the control mAb 11F11.