Abstract
Traditionally, platelets are known to play an important role in hemostasis, thrombosis, and wound healing, but increasing evidence suggests that activated platelets also may promote inflammation. Platelet-induced modulation of inflammation seems to involve platelet expression of ligands in the tumor necrosis factor (TNF) superfamily such as CD40 ligand and Fas ligand. The present study demonstrates that LIGHT, another member of the TNF superfamily, is associated with platelets and is released as a soluble ligand on platelet activation. The release of LIGHT involves GP IIb/IIIa-dependent mechanisms and action of metal-dependent proteases as well as intracellular processes such as actin polymerization. We also report that platelet-derived LIGHT is biologically active and can induce an inflammatory response in monocytes and particularly within endothelial cells measured as up-regulation of adhesion molecules and release of chemokines. Moreover, we demonstrate that thrombus material, obtained at the site of plaque rupture in patients with acute myocardial infarction, contains platelet-associated LIGHT, suggesting that LIGHT-mediated inflammation also is operating in vivo within an inflamed and thrombotic vessel wall. The data may suggest a pathogenic role for platelet-derived LIGHT in atherogenesis and plaque destabilization as well as in other inflammatory disorders involving leukocyte infiltration into the vessel wall.
Introduction
The traditional role of platelets as mediators of hemostasis and thrombosis is well documented. Increasing evidence suggests that activated platelets also play a key role in inflammation. Hence, on activation platelets release and express inflammatory mediators, induce an inflammatory response in adjacent leukocytes and endothelial cells, and respond with activation to several of the mediators produced by these cells.1-6 Such interactions between platelets and leukocytes/endothelial cells seem to play a pathogenic role in atherosclerosis as well as in other immune-mediated disorders.
Several platelet-derived mediators, such as chemokines and prostaglandins, appear to be involved in platelet-mediated inflammation.1 Recently, much attention has been focused on the role of platelet-associated CD40 ligand (CD40L), a ligand in the tumor necrosis factor (TNF) superfamily, in this inflammatory loop between platelets and other cells. Thus, platelet-associated CD40L may interact with CD40, which is constitutively expressed on a wide range of cells, such as macrophages, endothelial cells, and vascular smooth muscle cells, resulting in various inflammatory responses.7,8 Moreover, platelet-derived FasL, another member of the TNF superfamily, was recently shown to induce apoptosis in Fas+ tumor cells, further suggesting a role for ligands of this cytokine superfamily in platelet-mediated immune responses.9
LIGHT, the name of which is derived from “homologous to lymphotoxins, exhibits inducible expression, and competes with herpes simplex virus (HSV) glycoprotein D for herpes virus entry mediator (HVEM/TR2), a receptor expressed by T lymphocytes,” is a 29-kDa type 2 transmembrane protein also belonging to the TNF superfamily.10 LIGHT is produced by activated T cells, monocytes, granulocytes, and immature dendritic cells, and studies in animal models indicate that this cytokine may be crucial for the development of various autoimmune disorders (eg, inflammatory bowel disease, nephritis, and rheumatoid arthritis) through effects on T cells and T-cell homing into inflamed tissues.11-13 Moreover, LIGHT has also been suggested to promote atherogenesis at least partly by inducing matrix metalloproteinase (MMP) activity in macrophages.14
Because of the expression of certain TNF superfamily members by activated platelets, we investigated whether LIGHT is associated with platelets, and herein we show that these cells express LIGHT and that on activation they release significant amounts of this cytokine. We also report that platelet-derived LIGHT is biologically active and can induce an inflammatory response within endothelial cells and monocytes. The release of LIGHT from activated human platelets, shown here, emphasizes the importance of platelets in biologic processes beyond hemostasis and thrombus formation.
Materials and methods
Platelet preparation and stimulatio
Preparation and stimulation of citrated platelet-rich plasma (PRP) were performed as previously described.15 Briefly, PRP was incubated at 22°C with 100 μM SFLLRN (synthesized at the Biotechnology Centre of Oslo, Norway) or Tris-buffered saline (TS, pH 7.4; 20 mM Tris and 150 mM NaCl) only. At different time points, aliquots were removed and centrifuged at 13 000g for 5 minutes to obtain platelet-free plasma (PFP), which was stored at –80°C until LIGHT measurements. In a separate experiment, PFP was further centrifuged at 147 000g for 2 hours at 15°C to remove any microparticles. When testing the effect of a glycoprotein (GP) IIb/IIIa antagonist (abciximab; Eli Lily, Oslo, Norway) with 0.15 M sodium chloride as a control, or aspirin (Sigma, St Louis, MO) with methanol as a control, the substances were added 10 and 20 minutes prior to SFLLRN stimulation, respectively. When testing the effect of ethylenediaminetetraacetic acid (EDTA; Merck, Darmstadt, Germany), cytochalasin D (Sigma), and the MMP inhibitor GM6001 (Calbiochem, San Diego, CA), the inhibitors were added 10 minutes after SFLLRN activation. In a separate set of experiments, we also examined LIGHT levels in platelet pellets by analyzing the concentration in platelet lysates after lysing platelet pellets by adding a 1% Triton-lysis buffer supplemented with protease inhibitors (Complete Protease Inhibitor Cocktail, Roche Diagnostic, Mannheim, Germany). In some experiments, PRP was mixed with Dynabeads to remove any potential contamination of other blood cells from the PRP suspension. Briefly, one-fourth volume of acid-citrate-dextrose (ACD, pH = 4.5; 85 mM trisodium citrate, 71.4 mM citric acid, and 111 mM glucose) was added to PRP prior to centrifugation at 1500g for 7 minutes at 22°C and the pellet was resuspended in PBS containing 0.1% BSA and 2 mM EDTA. An antibody mix from the Monocyte Negative Isolation Kit (Dynal, Oslo, Norway) was used to mark T and B cells, natural killer (NK) cells, erythrocytes, and granulocytes if present. After a wash step, Dynabeads directed against the antibody-coated cells in combination with Dynabeads M-450 CD14 (Dynal) directed against monocytes were added to the platelet suspension, and after incubation of the mixture on a rocking platform for 30 minutes, any potential rosetting cells were removed using a magnetic particle concentrator. The platelet suspension was then either used for further in vitro experiments or centrifuged at 2000g for 10 minutes before storing (platelet pellets) at –80°C until RNA isolation. Flow cytometry analyses showed no CD45+ cells in the purified platelet suspension.
Flow cytometry
Flow cytometry analyses of platelets were performed as previously described.15 Briefly, unstirred PRP diluted 1:20 in autologous PFP was incubated at 22°C for 10 and 90 minutes with 100 μM SFLLRN or TS only. PRP was further incubated with the platelet-specific fluorescein isothiocyanate (FITC)–labeled antibody anti-CD41 (Dako, Glostrup, Denmark) and phycoerythrin (PE)–labeled antibody anti–human LIGHT/TNFSF14/CD258 (R&D Systems, Minneapolis, MN), or the unspecific PE-labeled antibody of the same isotype and quantity (mouse IgG1; Dako). In a separate experiment, platelets in diluted PRP were permeabilized with 0.05% saponin (Sigma) and, in addition to the mentioned antibodies, incubated with PE-labeled anti-CD62P (clone 1E3; Dako). A fluorescent-activated cell sorting (FACScan) flow cytometer (Becton Dickinson, San Jose, CA) was used and data from 10 000 cells were analyzed each time using the CellQuest program (Becton Dickinson).
Isolation and stimulation of monocytes
Monocytes were isolated from peripheral blood mononuclear cells as previously described,16 and incubated in flat-bottomed 96-well trays (106/mL; Costar, Cambridge, MA), in medium alone (RPMI 1640 with 2 mM l-glutamine [Gibco, Grand Island, NY]) supplemented with 10% FCS (Sigma) or stimulated with different concentrations of recombinant human LIGHT (rhLIGHT; R&D Systems) or platelet releasate (see “Incubation of HUVECs and monocytes with extracts of resting platelets or releasates from stimulated platelets”). Cell-free supernatants were harvested after 20 hours and stored at –80°C until analysis.
Endothelial cell culture
Human umbilical vein endothelial cells (HUVECs) were obtained from umbilical cord veins by digestion with 0.1% collagenase A (Boehringer Mannheim, Mannheim, Germany) as previously described.17 HUVECs were cultured in MCDB-131 media (Sigma) supplemented with 7.5% fetal bovine serum (Gibco), 250 ng/mL amphotericin B (Gibco), 50 μg/mL gentamicin (Gibco), 1 ng/mL basic fibroblast growth factor (R&D Systems), 10 ng/mL human epidermal growth factor (R&D Systems), and 1 μg/mL hydrocortisone (Sigma). HUVECs were passaged by treatment with 0.05% trypsin-EDTA (Gibco) and grown in 12-, 24-, or 96-well plates (Costar) to confluence for 3 to 5 days. The medium was then discarded, and HUVECs were stimulated with different concentrations of rhLIGHT (R&D Systems), extracts of resting platelets, or platelet releasates from stimulated platelets for 5 and 20 hours. In a separate experiment, HUVECs were preincubated for 20 minutes with ortho-hydroxy atorvastatin (gift from Pfizer, Sandwich, England) with and without mevalonate (Sigma) before stimulation with rhLIGHT. The endotoxin levels of all stimulants and culture media were less than 10 pg/mL (Limulus Amebocyte Assay; BioWhittaker, Walkersville, MD).
Incubation of HUVECs and monocytes with extracts of resting platelets or releasates from stimulated platelets
Preparation of extracts from resting platelets or releasates from stimulated platelets was performed by adding one-fourth volume of ACD to PRP prior to centrifugation at 1500g for 7 minutes at 22°C. The platelets were then resuspended in MCDB-131 media (Sigma, 109 platelets/mL) before either being exposed to 3 freeze-and-thaw cycles or stimulated with 0.1 U/mL thrombin (Sigma) for 10 and 90 minutes to induce release of platelet components to the media. Hirudin (0.4 U/mL, Sigma) was added to neutralize thrombin. The platelets were then removed by centrifugation at 13 000g for 5 minutes at 22°C and the supernatants representing platelet extracts and platelet releasates, respectively, were then added to confluent HUVECs or monocytes (only releasate), and in some experiments with LIGHT-neutralizing (TNFSF14; R&D System) or an irrelevant (MOPC-21, Sigma) antibody for 5 or 20 hours, respectively. According to the manufacturer the LIGHT antibody has been tested against all other known TNF superfamily ligands without showing any cross-reactivity.
Real-time quantitative reverse transcription-polymerase chain reaction
Total RNA was extracted from platelets using the MagNa Pure LC Instrument (Roche Applied Science, Indianapolis, IN). Primers for LIGHT (forward primer [FP]: 5′-CGTCCGTGTGCTGGATGA-3′ and reverse primer [RP]: 5′-ACCATGAAAGCCCCGAAGTA-3′ and β-actin FP: 5′-AGGCACCAGGGCGTGAT-3′ and RP: 5′-TCGTCCCAGTTGGTGACGAT-3′) were designed using the Primer Express software version 1.5 (Applied Biosystems, Foster City, CA).18
RNase protection assay
Total RNA was extracted from HUVECs using RNeasy columns (Qiagen, Hilden, Germany) and stored in RNA storage solution (Ambion, Austin, TX) at –80°C. The RNase protection assay (RPA) was performed with the chemokine (hCK5) multiprobe (PharMingen, San Diego, CA).19 Gene expression of the housekeeping gene GAPDH was used for normalization.
Cellular ELISA
Total cellular expression of E-selectin and vascular cellular adhesion molecule 1 (VCAM-1) was measured by an enzyme-linked immunosorbent assay (ELISA) on fixed adherent HUVECs as previously described.20 In brief, confluent monolayers of HUVECs, grown and stimulated in 96-well trays (Costar), were washed with PBS and fixed in fresh 0.5% periodatelysine-paraformaldehyde buffer for 10 minutes at 22°C. The cells were then dried thoroughly with cold air and permeabilized with 0.1% saponin (Sigma) in PBS for 10 minutes. Subsequently, the fixed cells were incubated with relevant monoclonal antibody (0.33 μg/mL anti–human E-selectin or 1 μg/mL VCAM-1 and isotype-matched controls; all from R&D Systems) for 45 minutes under constant shaking at 22°C followed by 3 washes with PBS. Secondary peroxidase-conjugated antibody (0.4 μg/mL; Pierce Biotechnology, Rockford, IL) was applied in the same manner followed by 4 washes with PBS. Tetramethylene benzidine substrate solution (100 μL/well; Zymed, San Francisco, CA) was added and developed under constant shaking at 22°C for 5 to 10 minutes. The color reaction was stopped with 1 M H2SO4 (100 μL/well) and OD was read in a Multiskan Ascent (Thermo Labsystems, Helsinki, Finland).
Measurements of cytokines by ELISA
Concentrations of soluble LIGHT, monocyte chemoattractant peptide 1 (MCP-1), and interleukin 8 (IL-8) were measured by ELISAs obtained from R&D Systems.
Patients
In a separate series of experiments, we analyzed LIGHT levels in PFP from 20 patients (59.5 ± 5 years, 8 women and 12 men) with unstable angina defined as ischemic chest pain at rest within the preceding 48 hours, as previously described.21 We also analyzed LIGHT levels in PFP from 1 woman and 8 men (62 ± 4 years) with stable angina21 undergoing percutaneous coronary intervention (PCI). In another experiment, we analyzed the release of LIGHT from platelets of one person previously diagnosed as having Glanzmann thrombasthenia showing less than 2% of the aggregation receptor GP IIb/IIIa compared to a healthy control analyzed simultaneously (flow cytometry).
Tissue sampling of thrombus materials
In 8 patients with acute ST-elevation myocardial infarction (STEMI) undergoing primary PCI, thrombus material at the site of the occlusion was aspirated immediately after crossing the lesion with the guidewire. A monorail aspiration catheter (Pronto, Vascular Solutions, Minneapolis, MN) was advanced over the wire, and a 20-mL air-filled syringe was used to aspirate during advancing the catheter through the occluded segment. The catheter was pushed and pulled through the segment during continuous aspiration until the syringe was filled with no vacuum left. The aspiration catheter was removed, and solid thrombus material was separated from liquid blood by means of a sieve (pore filter size, 40 μm; Pronto). The solid material was fixed in 4% paraformaldehyde and embedded in paraffin.
Immunohistochemistry
Paraformaldehyde-fixed sections of thrombus material were stained using monoclonal mouse anti–human LIGHT/TNFSF14 (R&D Systems) and mouse anti–human CD41 (Immunotech, Marseille, France) antibodies and affinity-purified polyclonal mouse anti–human monocytes/macrophages (calprotectin) IgG (MCA874G, Serotec, Oxford, United Kingdom). The primary antibodies were followed by biotinylated anti–mouse IgG (Vector Laboratories, Burlingame, CA). The immunoreactivities were further amplified using avidin-biotin-peroxidase complexes (Vectastain Elite kit, Vector Laboratories). Diaminobenzidine was used as the chromogen in a commercial metal-enhanced system (Pierce Chemical, Rockford, IL). The sections were counterstained with hematoxylin. Omission of the primary antibody served as a negative control. Images were obtained using an Axioskop 2 microscope with a 40×/0.75 NA oil objective and AxioCam camera with Axio Vision 3.0 software (Carl Zeiss, Jena, Germany).
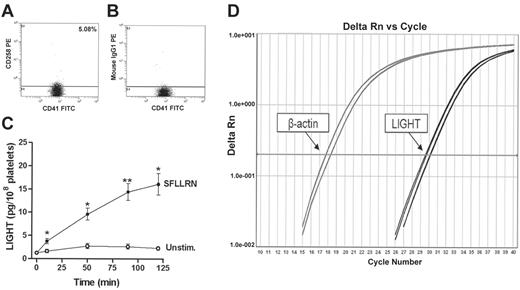
Expression and release of LIGHT from platelets. Flow cytometry analyses of LIGHT detected on resting platelets (gated by anti-CD41) measured as binding of the LIGHT-specific antibody anti-CD258 (A) compared with the binding of an unspecific antibody (B) with the same isotype and concentration. The horizontal line represents the separation of negative and positive events. A representative of 5 experiments is shown. Panel C shows the amount of released LIGHT (pg/108 platelets) measured in the extracellular phase after activation of platelets in PRP by SFLLRN (100 μM). Release from stimulated (•) and unstimulated (○) platelets. Data are presented as mean ± SEM, n = 5. *P < .05 and **P < .01 versus unstimulated platelets at the same time point. Panel D shows amplification plots (real-time PCR) demonstrating gene expression of LIGHT in platelets. Gene expression of β-actin is shown for comparison.
Expression and release of LIGHT from platelets. Flow cytometry analyses of LIGHT detected on resting platelets (gated by anti-CD41) measured as binding of the LIGHT-specific antibody anti-CD258 (A) compared with the binding of an unspecific antibody (B) with the same isotype and concentration. The horizontal line represents the separation of negative and positive events. A representative of 5 experiments is shown. Panel C shows the amount of released LIGHT (pg/108 platelets) measured in the extracellular phase after activation of platelets in PRP by SFLLRN (100 μM). Release from stimulated (•) and unstimulated (○) platelets. Data are presented as mean ± SEM, n = 5. *P < .05 and **P < .01 versus unstimulated platelets at the same time point. Panel D shows amplification plots (real-time PCR) demonstrating gene expression of LIGHT in platelets. Gene expression of β-actin is shown for comparison.
Ethics
In the studies involving humans, informed consent for participation was obtained from all individuals. The studies were conducted according to the ethical guidelines at our hospital according to the Declaration of Helsinki and were approved by the hospital's authorized representative.
Statistical analysis
For statistical analysis of the in vitro experiments, the Student paired t test was used. For comparisons of 2 groups of individuals, the Mann-Whitney rank sum test was used. For comparisons within the same individuals, the Wilcoxon matched pairs test was used. P values (2-sided) were considered significant at levels below .05.
Results
Release of LIGHT from activated platelets
Based on previous data showing expression of TNF superfamily proteins (ie, CD40L, FasL, and TRAIL) in platelets,7,9,22 we wanted to determine whether these cells also contain LIGHT. As shown in Figure 1A-B, 5% of resting platelets expressed LIGHT on their surface as assessed by flow cytometry comparing the binding of a specific antibody against LIGHT with its isotype antibody control. Notably, we could not detect any further increase in surface exposure of LIGHT after platelet activation with SFLLRN (100 μM) for 10 or 90 minutes. We also examined the expression of LIGHT inside the platelets after permeabilization with 0.05% saponin. Although we could not detect any further increase in LIGHT fluorescence after permeabilization, this process resulted in a marked increase in the proportion of platelets that bound the P-selectin antibody (75.8%). These data may suggest that the amount of intracellular LIGHT is markedly lower than the amount of P-selectin and perhaps close to the detection limit of the assay. However, our findings may also reflect that LIGHT is differentially stored and released than those substances that are regulated through an ordinary α-granule release. In contrast to membrane-bound LIGHT, platelets gradually released soluble LIGHT on SFLLRN activation (100 μM), reaching a maximum after 120 minutes as assessed by ELISA measurements in PFP (Figure 1C). This long-lasting release, which follows the same pattern as CD40L solubilization from platelets,15 differs from an ordinary α-granule release where the granule components are fully released within less than 10 minutes,15,23,24 further indicating an alternative way of LIGHT release from platelets than direct release from α-granules.
Although plasma levels of LIGHT in healthy controls (∼15 pg/ml)25 are much lower than the LIGHT levels found in SFLLRN-activated PRP, we cannot exclude the possibility that LIGHT is adsorbed to the platelet surface in circulation or as a consequence of the ex vivo procedure of the blood samples. To further verify that LIGHT is a platelet-derived cytokine, we therefore performed additional experiments. It is well known that platelets retain a small but functionally significant amount of megakaryocyte-derived RNA as well as the proteins and molecular machinery necessary for translation26 and, indeed, by using real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR), we showed expression of mRNA for LIGHT in purified platelets (Figure 1D). Moreover, by using purified platelets (see “Materials and methods”) we found the same pattern of LIGHT release as observed in SFLLRN-stimulated PRP (data not shown). Finally, we found no differences in LIGHT concentration between supernatants centrifuged at “normal speed conditions” (ie, 13 000g for 5 minutes) and supernatants exposed to ultracentrifugation (ie, 147 000g for 2 hours), suggesting that LIGHT in activated PRP is truly soluble and not bound to microparticles. Although further studies are needed to fully clarify the mechanisms of LIGHT release from platelets, these findings suggest that LIGHT is a platelet-derived cytokine.
Involvement of metal-dependent proteases in the release of LIGHT from platelet
Members of the TNF superfamily have been reported to be cleaved by calcium-dependent MMPs and by a family of disintegrin and metalloproteinases (ADAMs), both of which are inhibited by hydroxamate inhibitors of MMPs.27 Therefore, to further examine the mechanism of LIGHT release from platelets, we assessed the effects of EDTA (a strong calcium chelator) and GM6001 (a hydroxamate inhibitor of MMPs). In addition, we assessed the effects of cytochalasin D (an inhibitor of actin polymerization). As shown in Figure 2A, all these inhibitors markedly reduced the SFLLRN-stimulated release of LIGHT when added 10 minutes after SFLLRN stimulation. Our findings so far suggest that the gradual release of LIGHT from SFLLRN-activated platelets involves metal-dependent proteases as well as intracellular processes such as actin polymerization showing a similar pattern as for the release of soluble (s) CD40L.15,23,28,29
Effect of different platelet inhibitors and GP IIb/IIIa antagonists on plasma levels of LIGHT. (A) Release of LIGHT from platelets in PRP (after 90 minutes of activation), in the presence of EDTA (5 mM), cytochalasin D (60 μM), or GM 6001 (30 μM) added to PRP 10 minutes after the activation agonist SFLLRN (100 μM), compared to stimulated platelets with solvent added instead of inhibitor. Data are presented as mean ± SEM, n = 5. (B) Release of LIGHT (pg/108 platelets) measured in the extracellular phase after activation of platelets in PRP by SFLLRN (100 μM) from stimulated platelets (•), from platelets preincubated (for 10 minutes) with abciximab (▴; 40 μg/mL), and from platelets obtained from a patient (▪) with Glanzmann thrombasthenia (lacking the aggregation receptor GP IIb/IIIa). Data are presented as mean ± SEM; n = 10, n = 5 and n = 1, respectively. *P < .05 versus no inhibitor (panel A) and versus unstimulated platelets at the same point of time (panel B). (C) Release of LIGHT from platelets in PRP stimulated with 100 μM SFLLRN (for 90 minutes) from patients with unstable angina with (n = 10) and without (n = 10) anti–GP IIb/IIIa therapy. (D) LIGHT in platelet pellets (at baseline) obtained from the same patient groups. Data are presented as mean ± SEM, **P < .01 and ***P < .001 versus patients without anti–GP IIb/IIIa therapy. (E) Release of LIGHT from platelets in PRP after preincubation with aspirin (1 mM) for 20 minutes and stimulation with SFLLRN (100 μM) for 90 minutes. (F) Effect of aspirin (160 mg once a day, for 7 days) on plasma levels of LIGHT in 12 healthy controls.
Effect of different platelet inhibitors and GP IIb/IIIa antagonists on plasma levels of LIGHT. (A) Release of LIGHT from platelets in PRP (after 90 minutes of activation), in the presence of EDTA (5 mM), cytochalasin D (60 μM), or GM 6001 (30 μM) added to PRP 10 minutes after the activation agonist SFLLRN (100 μM), compared to stimulated platelets with solvent added instead of inhibitor. Data are presented as mean ± SEM, n = 5. (B) Release of LIGHT (pg/108 platelets) measured in the extracellular phase after activation of platelets in PRP by SFLLRN (100 μM) from stimulated platelets (•), from platelets preincubated (for 10 minutes) with abciximab (▴; 40 μg/mL), and from platelets obtained from a patient (▪) with Glanzmann thrombasthenia (lacking the aggregation receptor GP IIb/IIIa). Data are presented as mean ± SEM; n = 10, n = 5 and n = 1, respectively. *P < .05 versus no inhibitor (panel A) and versus unstimulated platelets at the same point of time (panel B). (C) Release of LIGHT from platelets in PRP stimulated with 100 μM SFLLRN (for 90 minutes) from patients with unstable angina with (n = 10) and without (n = 10) anti–GP IIb/IIIa therapy. (D) LIGHT in platelet pellets (at baseline) obtained from the same patient groups. Data are presented as mean ± SEM, **P < .01 and ***P < .001 versus patients without anti–GP IIb/IIIa therapy. (E) Release of LIGHT from platelets in PRP after preincubation with aspirin (1 mM) for 20 minutes and stimulation with SFLLRN (100 μM) for 90 minutes. (F) Effect of aspirin (160 mg once a day, for 7 days) on plasma levels of LIGHT in 12 healthy controls.
Effect of GP IIb/IIIa antagonism on the platelet release of LIGH
GP IIb/IIIa antagonists block the binding of soluble fibrinogen to the activated GP IIb/IIIa complex on the platelet surface, thereby inhibiting platelet aggregation.29 In addition, we and others have reported that GP IIb/IIIa antagonists are able to inhibit the release of sCD40L from activated platelets.15,28,29 The ability of these antagonists to inhibit LIGHT release from platelets was therefore examined. First, we found that the GP IIb/IIIa antagonist abciximab (40 μg/mL) totally abolished the release of LIGHT from SFLLRN-activated platelets when added to PRP 10 minutes prior to activation (Figure 2B). Second, a similar pattern was seen in SFLLRN-stimulated platelets from a patient with Glanzmann thrombasthenia, an inherited deficiency of GP IIb/IIIa on platelets (Figure 2B). Finally, when examining platelets from patients with unstable angina with (n = 10) and without (n = 10) anti–GP IIb/IIIa therapy, we found that platelets from the former group were characterized by decreased SFLLRN-mediated release of LIGHT and increased amount of LIGHT in platelet pellets before stimulation, suggesting that the GP IIb/IIIa-mediated inhibition of platelet LIGHT release also is operating in vivo (Figure 2C-D). Whether this attenuating effect of GP IIb/IIIa inhibition on LIGHT release was achieved because of inhibition of aggregation or if it reflects a direct action of abciximab on platelets (ie, attenuated fibrinogen binding to activated GPIIb/IIIa) will have to be further clarified.
Effect of aspirin on the platelet release of LIGHT
Aspirin, inhibiting COX-1, also blocks CD40L release from platelets,29 and as shown in Figure 2E, aspirin significantly reduced the SFLLRN-induced release of LIGHT in PRP. Moreover, when examining the effect of aspirin in the in vivo situation, we found that this medication (160 mg once a day) significantly down-regulated plasma levels of LIGHT when given for 7 days in 12 healthy controls (Figure 2F).
Effects of rhLIGHT on HUVECs
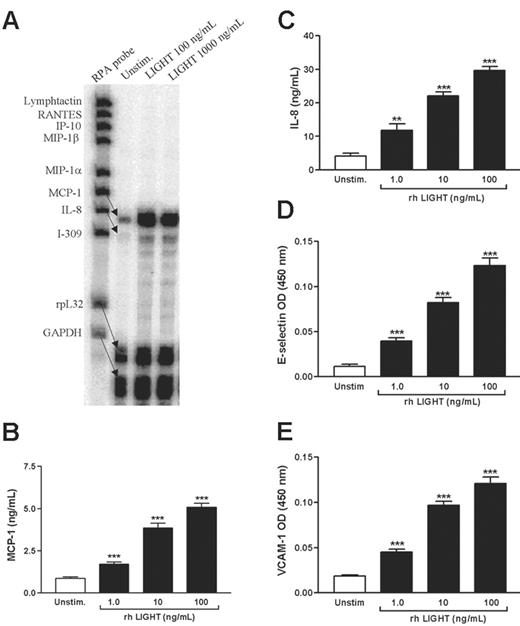
Endothelial cell activation is an important feature of several inflammatory disorders, including atherosclerosis, and to elucidate any inflammatory effects of platelet-derived LIGHT, we first examined the ability of rhLIGHT to enhance the expression of chemokines in HUVECs. Using RPA, we screened for the expression of 8 different CC and CXC chemokines, and as shown in Figure 3A, rhLIGHT selectively induced MCP-1 and IL-8 gene expression. This effect of rhLIGHT on endothelial cells was also verified at the protein level, showing a dose-dependent pattern as assessed by ELISA measurements in HUVEC supernatants (Figure 3B-C). In addition to the induction of chemokines, up-regulation of adhesion molecules on endothelial cells is of major importance for the mobilization of leukocytes into inflamed tissue,30 and notably, rhLIGHT markedly and dose dependently enhanced the protein levels of E-selectin and VCAM-1 in HUVECs as assessed by cellular ELISA (Figure 3D-E).
Effect of platelet-derived LIGHT on HUVECs
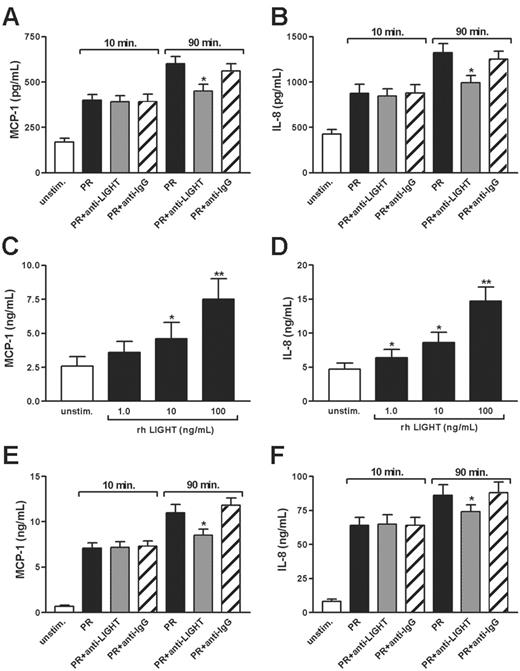
To see whether the rhLIGHT-mediated effects on endothelial cell-related inflammation also could be induced by platelet-derived LIGHT, we incubated HUVECs with both extracts from resting platelets and releasates from thrombin-stimulated (0.1 U/mL) platelets. As shown in Figure 4A-B, platelet releasates from platelets stimulated for 10 and 90 minutes markedly up-regulated the release of MCP-1 and IL-8 in HUVECs. More importantly, whereas an unspecific antibody had only a minor effect on the release of these chemokines, a neutralizing antibody against LIGHT markedly reduced the levels of IL-8 and MCP-1 in HUVECs when platelets were stimulated with thrombin for 90 minutes before adding platelet releasates to HUVECs (Figure 4A-B). The same pattern was observed when stimulating HUVECs with platelet extracts (data not shown). Interestingly, the neutralizing antibody against LIGHT did not influence the enhancing effect of platelet releasates from thrombin-stimulated platelets on chemokine levels in HUVECs when platelets were stimulated for 10 minutes, further illustrating the slow kinetics of LIGHT release from activated platelets (Figure 4A-B). Taken together, these data clearly suggest that soluble LIGHT released from activated platelets is biologically active.
Chemokine and adhesion molecules expression in HUVECs stimulated with rhLIGHT. (A) RPA from one representative experiment (5 hours). GAPDH and rpL32 represent control genes. (B-E) Effect of rhLIGHT (1-100 ng/mL) on protein levels of MCP-1 (B) and IL-8 (C) in HUVEC supernatants (20 hours), and E-selectin (D) and VCAM-1 (E) associated with attached HUVECs (5 hours), n = 9 for both. Data are presented as mean ± SEM. **P < .01 and ***P < .001 versus unstimulated HUVECs.
Chemokine and adhesion molecules expression in HUVECs stimulated with rhLIGHT. (A) RPA from one representative experiment (5 hours). GAPDH and rpL32 represent control genes. (B-E) Effect of rhLIGHT (1-100 ng/mL) on protein levels of MCP-1 (B) and IL-8 (C) in HUVEC supernatants (20 hours), and E-selectin (D) and VCAM-1 (E) associated with attached HUVECs (5 hours), n = 9 for both. Data are presented as mean ± SEM. **P < .01 and ***P < .001 versus unstimulated HUVECs.
Effect of platelet-derived LIGHT on chemokine-release from HUVECs and monocytes. The diagrams show the ability of a neutralizing antibody against LIGHT (anti-LIGHT; 100 μg/mL) to attenuate the effect of platelet releasate (PR) prepared from thrombin-stimulated platelets (0.1 U/mL; for 10 and 90 minutes) on the enhancement of MCP-1 (A) and IL-8 (B) from HUVECs after 5 hours of stimulation and MCP-1 (E) and IL-8 (F) from monocytes after 20 hours of stimulation. A control antibody of the same isotype (IgG) and concentration was used as a control. Data are presented as mean ± SEM, n = 6. *P < .05 versus HUVECs or monocytes stimulated with platelet releasate. (C-D) Effect of rhLIGHT (1-100 ng/mL) on protein levels of MCP-1 (C) and IL-8 (D) in monocyte supernatants stimulated for 20 hours. Data are presented as mean ± SEM, n = 6. *P < .05 and **P < .01 versus unstimulated cells.
Effect of platelet-derived LIGHT on chemokine-release from HUVECs and monocytes. The diagrams show the ability of a neutralizing antibody against LIGHT (anti-LIGHT; 100 μg/mL) to attenuate the effect of platelet releasate (PR) prepared from thrombin-stimulated platelets (0.1 U/mL; for 10 and 90 minutes) on the enhancement of MCP-1 (A) and IL-8 (B) from HUVECs after 5 hours of stimulation and MCP-1 (E) and IL-8 (F) from monocytes after 20 hours of stimulation. A control antibody of the same isotype (IgG) and concentration was used as a control. Data are presented as mean ± SEM, n = 6. *P < .05 versus HUVECs or monocytes stimulated with platelet releasate. (C-D) Effect of rhLIGHT (1-100 ng/mL) on protein levels of MCP-1 (C) and IL-8 (D) in monocyte supernatants stimulated for 20 hours. Data are presented as mean ± SEM, n = 6. *P < .05 and **P < .01 versus unstimulated cells.
Effect of recombinant and platelet-derived LIGHT on monocytes
LIGHT has very recently been reported to enhance monocyte activation.31 To further elucidate this issue, we next examined the effects of rhLIGHT on the release of IL-8 and MCP-1 in monocytes from 5 healthy controls after culturing for 20 hours. As shown in Figure 4C-D, rhLIGHT dose dependently enhanced the release of these chemokines in monocytes although the increase was relatively modest (∼3-fold increase) compared with the LIGHT-mediated effects on HUVECs. Moreover, platelet releasates from thrombin-stimulated (0.1 U/mL) platelets markedly enhanced the release of IL-8 and MCP-1 from monocytes, with a significantly but moderately attenuating effect of neutralizing antibody against LIGHT (∼25%). However, such a neutralizing effect was seen only when platelets were stimulated with thrombin for 90 minutes, and not after stimulation for 10 minutes, before adding platelet releasates to monocytes, showing the same pattern as for the platelet-mediated effect on HUVECs (Figure 4E-F). Although our findings clearly suggest that recombinant as well as platelet-derived LIGHT could promote an inflammatory response in monocytes, the LIGHT-mediated effects on endothelial cells seem to be more prominent, at least in our hands.
Plasma levels of LIGHT after PCI
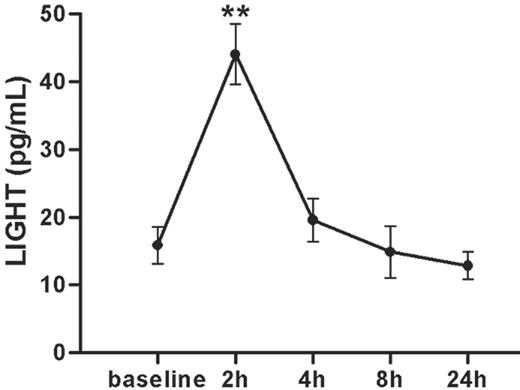
Platelet activation is an important feature of coronary artery disease (CAD) and plaque rupture and to further elucidate the clinical relevance of our findings, we examined plasma levels of LIGHT before and 2, 4, 8, and 24 hours after PCI in 9 patients with stable angina pectoris. PCI represents a human in vivo model for mechanically induced plaque rupture, and notably, this procedure induced a transient (after 2 hours) and marked increase (2–3-fold) in LIGHT levels in PFP, suggesting a relationship between LIGHT activation and plaque rupture (Figure 5).
Expression of LIGHT in arterial thrombosis
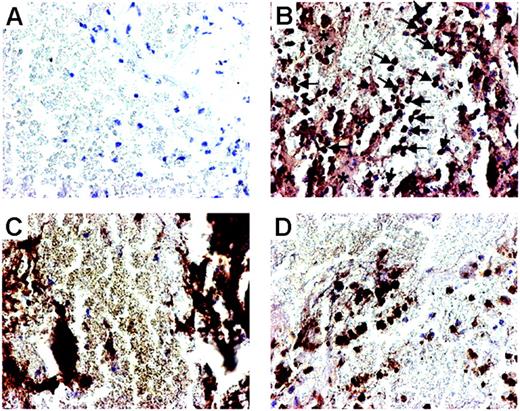
To further elucidate this issue, we examined the expression of LIGHT in thrombus material in aspirates obtained from 8 patients with acute STEMI undergoing primary PCI. Immunohistochemical staining of the thrombus material removed from the site of the ruptured plaque showed strong LIGHT immunostaining on platelets and monocytes/macrophages, further suggesting that platelet-associated LIGHT may be involved in plaque destabilization (Figure 6).
Effects of statins on LIGHT-induced activation of HUVECs
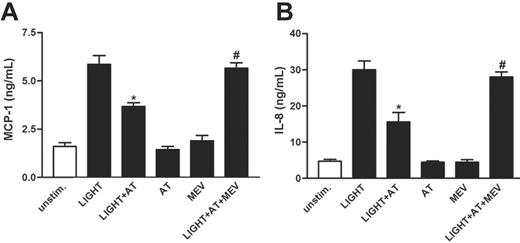
Our findings so far may suggest that platelet-derived LIGHT could contribute to endothelial activation in patients with CAD, possibly promoting plaque progression and destabilization. Numerous studies have demonstrated an improved prognosis in patients with CAD receiving HMG-CoA reductase inhibitors (statins), and although this effect has been attributed to their lipid-lowering potential, recent studies suggest that these drugs also could modulate the inflammatory arm of atherosclerosis.32 We therefore next examined whether statins could have any modulating effect on the LIGHT-induced activation of endothelial cells. Whereas the atorvastatin metabolite ortho-hydroxy atorvastatin had no effect on the release of MCP-1 and IL-8 when given alone, it significantly reduced the rhLIGHT-dependent release of these chemokines from HUVECs (Figure 7A-B). Moreover, mevalonate nearly abolished this suppressive effect of ortho-hydroxy atorvastatin on the rhLIGHT-induced chemokine release, suggesting that this statin-mediated effect involves inhibition of the mevalonate pathway (Figure 7A-B). These in vitro findings further underscore the anti-inflammatory potential of statins.
Plasma levels of LIGHT in patients with stable angina undergoing PCI. Plasma levels of LIGHT (pg/mL) in 9 patients with stable angina before (baseline) and at different time points after PCI. Data are presented as mean ± SEM, n = 9. **P < .01 versus baseline.
Plasma levels of LIGHT in patients with stable angina undergoing PCI. Plasma levels of LIGHT (pg/mL) in 9 patients with stable angina before (baseline) and at different time points after PCI. Data are presented as mean ± SEM, n = 9. **P < .01 versus baseline.
Localization of LIGHT in thrombus material. Photomicrographs showing immunostaining and localization of LIGHT in thrombus material removed from the site of plaque rupture in patients with STEMI undergoing PCI. Thrombus material stained with omission of the primary antibody served as control and demonstrated no immunostaining of any of the cellular elements (A). Fairly strong immunostaining of LIGHT (B) was seen in areas with platelets (*) and in monocytes/macrophages (arrows). Panel C demonstrates thrombus material with CD41+platelets, and panel D demonstrates calprotectin-positive monocytes/macrophages. Original magnification ×400.
Localization of LIGHT in thrombus material. Photomicrographs showing immunostaining and localization of LIGHT in thrombus material removed from the site of plaque rupture in patients with STEMI undergoing PCI. Thrombus material stained with omission of the primary antibody served as control and demonstrated no immunostaining of any of the cellular elements (A). Fairly strong immunostaining of LIGHT (B) was seen in areas with platelets (*) and in monocytes/macrophages (arrows). Panel C demonstrates thrombus material with CD41+platelets, and panel D demonstrates calprotectin-positive monocytes/macrophages. Original magnification ×400.
Discussion
Recent studies have demonstrated that platelets express certain members of the TNF superfamily,7,9,22 and the present study shows that LIGHT, another member of this family, should be added to the list of inflammatory mediators that are released from platelets on activation. Moreover, in contrast to the rapid release of α-granule contents, activated platelets release LIGHT in a gradual and long-lasting manner, involving GP IIb/IIIa-dependent mechanisms, showing a similar pattern as previously has been described for platelet release of sCD40L.15,28,29 The ability of platelet-derived LIGHT to induce inflammation in endothelial cells and monocytes, and the fact that platelets at the site of atherosclerotic plaque rupture express this cytokine, suggest a pathogenic role for platelet-derived LIGHT in atherogenesis and plaque destabilization as well as in other inflammatory disorders involving leukocyte infiltration into the vessel wall. The recent report of increased plasma levels of LIGHT in angina patients, with particularly high levels in those with unstable disease,25 further supports such a notion. Our data may also suggest that the beneficial effects of aspirin, GP IIb/IIIa antagonists, and a statin in CAD could involve inhibition of LIGHT-mediated inflammation.
The effect of atorvastatin on chemokine-release from HUVECs. HUVECs were preincubated (for 20 minutes) with ortho-hydroxy atorvastatin (AT; 10 μM) with and without mevalonate (MEV; 100 μM) before stimulation with rhLIGHT (100 ng/mL) for 5 hours. Atorvastatin reduced the rhLIGHT-mediated release of MCP-1 (A) and IL-8 (B). Data are presented as mean ± SEM, n = 6. *P < .05 versus stimulated HUVECs with solvent added instead of atorvastatin. #P < .05 shown for stimulated HUVECs preincubated with mevalonate and atorvastatin versus stimulated HUVECs preincubated with atorvastatin only.
The effect of atorvastatin on chemokine-release from HUVECs. HUVECs were preincubated (for 20 minutes) with ortho-hydroxy atorvastatin (AT; 10 μM) with and without mevalonate (MEV; 100 μM) before stimulation with rhLIGHT (100 ng/mL) for 5 hours. Atorvastatin reduced the rhLIGHT-mediated release of MCP-1 (A) and IL-8 (B). Data are presented as mean ± SEM, n = 6. *P < .05 versus stimulated HUVECs with solvent added instead of atorvastatin. #P < .05 shown for stimulated HUVECs preincubated with mevalonate and atorvastatin versus stimulated HUVECs preincubated with atorvastatin only.
As for the mechanism of LIGHT release from platelets, the present study suggests that the GP IIb/IIIa complex is important for this process. Thus, Glanzmann thrombasthenia platelets and normal platelets treated with a fibrinogen-blocking GP IIb/IIIa antagonist showed markedly reduced activation-dependent LIGHT release. A similar pattern was also seen during GP IIb/IIIa inhibitory therapy in vivo, with a decrease in SFLLRN-mediated LIGHT release ex vivo in PRP from patients with unstable angina receiving GP IIb/IIIa antagonist therapy as compared with those who did not. These findings may indicate that maximal release of LIGHT requires cell-to-cell contact and that this is prevented in Glanzmann thrombasthenia and in the presence of GP IIb/IIIa antagonists, or that an outside-in signaling due to binding of fibrinogen to the activated GPIIb/IIIa complex may be important in this respect. Because GPIIb/IIIa does not exist on other blood cells than platelets, the decrease in LIGHT levels in SFLLRN-activated PRP in the presence of abciximab also further supports the notion of the platelet as the source of LIGHT in the PRP experiments. It has been suggested that the long-term benefit of GP IIb/IIIa antagonists could be due not only to the inhibition of thrombus formation, but also to attenuated platelet-mediated inflammation.33 Our finding in the present study, showing a strong inhibitory effect of GP IIb/IIIa antagonists on the release of LIGHT, both in vivo and in vitro, further supports such anti-inflammatory effects of these medications as far as an accumulation of active LIGHT on the platelets does not occur.
Cytochalasin D, which is known to inhibit actin polymerization and disrupt actin microfilaments and therefore may be expected to affect endocytotic and exocytotic transport processes, reduced the release of LIGHT from activated platelets. Further, the MMP inhibitor GM6001, which potentially can act both intracellularly and on the outer platelet membrane, and EDTA, which may neutralize metal-dependent enzymes on the outer membrane, both reduced the release of LIGHT from activated platelets. These results may implicate intracellular structures and reactions to be important for the release of LIGHT, and suggest that LIGHT, at least partly, is released from the platelets due to the action of metal-dependent enzymes. Our results also demonstrate that LIGHT, like sCD40L, is released differently from the regular secretion from α-granules, which is ended within 10 minutes after stimulation. Whether such a gradual and long-lasting release, as shown for LIGHT, will result in a sustained inflammatory response in adjacent cells needs further investigation.
Up-regulation of adhesion molecules and chemokines is an important step in the recruitment and activation of leukocytes into inflamed tissues. Herein we show that LIGHT is a potent inducer of the adhesion molecules E-selectin and VCAM-1 as well as the chemokines MCP-1 and IL-8 in endothelial cells, and LIGHT was also found to induce chemokine release in monocytes. Importantly, these enhancing effects were not only seen in our experiments using rhLIGHT. In fact, although the amount of platelet-derived LIGHT is relatively small as compared to, for example, sCD40L (ie, picogram versus nanogram levels), the neutralizing antibody against LIGHT significantly attenuated the platelet-mediated enhancing effect of MCP-1 and IL-8, suggesting that platelet-derived LIGHT also possesses inflammatory properties. Furthermore, although we previously have shown enhancing effects of rhCD40L on the release of IL-8 and MCP-1 only at a concentration above 1 μg/mL,34 similar effects of rhLIGHT were seen at a concentration of 1 ng/mL, further underscoring that soluble LIGHT is a potent inducer of platelet-mediated inflammation in monocytes and particularly in endothelial cells. Finally, by immunohistochemistry we demonstrate that platelets in patients with acute myocardial infarction express LIGHT in thrombus material obtained at the site of plaque rupture, suggesting that such LIGHT-mediated inflammation also is operating in vivo within an inflamed and thrombotic vessel wall.
In conclusion, although further studies are needed to fully clarify the mechanisms of LIGHT release from platelets, our data suggest that LIGHT is a platelet-derived protein and that on activation these cells release significant amounts of this cytokine. The release of LIGHT is a long-lasting process and seems to be differently regulated than the membrane exposure of this cytokine. We also report that platelet-derived LIGHT is biologically active and induces inflammatory responses in endothelial cells and monocytes with particularly potent effects within endothelial cells. Of note, our studies of thrombus material from patients with acute myocardial infarction suggest that such LIGHT-mediated inflammation also is operating in vivo. These data expand the spectrum of TNF superfamily proteins that are expressed in platelets and suggest a pathogenic role for platelet-derived LIGHT in atherogenesis and plaque destabilization as well as in other inflammatory disorders involving leukocyte infiltration into the vessel wall.
Prepublished online as Blood First Edition Paper, April 13, 2006; DOI 10.1182/blood-2005-09-010629.
Supported by the Norwegian Council on Cardiovascular Diseases, Professor Paul A. Owren's Fund, Research Council of Norway including the functional genomics program (FUGE), the University of Oslo, and the Medinnova Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.