Comment on Rudelius et al, page 1668
Patients with mantle cell lymphoma (MCL) generally have a poor prognosis. Rudelius and colleagues now show that the PI 3-kinase/Akt prosurvival signaling pathway is constitutively activated in the more aggressive blastoid form of MCL and that inhibitory drugs targeting this pathway promote the apoptosis of MCL cells, raising the possibility of new treatments for blastoid MCL.
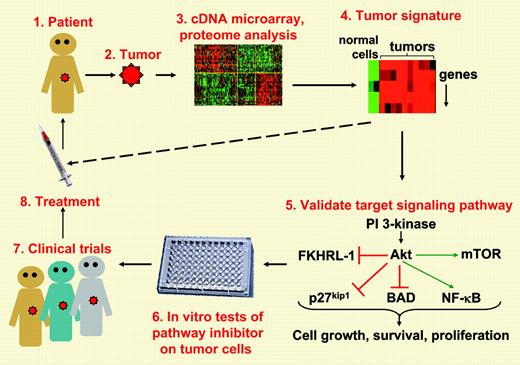
Ever since Staudt1 and colleagues pioneered the use of cDNA microarrays to develop molecular signatures for distinct types of lymphomas, molecular oncologists have been using this approach to identify signaling pathways that are critical for tumor survival and, hence, targets for anticancer drugs (see figure). In this issue, Rudelius and colleagues suggest that targeting the PI 3-kinase (PI3K)/Akt prosurvival pathway may be useful for treating mantle cell lymphoma (MCL). MCL is a generally incurable B-cell lymphoma with median survival times of 3 to 5 years. In approximately 20% of cases, patients develop a more aggressive blastoid form of MCL. Gene expression profiling had suggested that PI3K and its downstream target, the Akt protein kinase, were overexpressed in MCLs.3 However, functional data indicating that this signaling pathway was dysregulated in MCL, and contributed to the survival of MCL cells, was lacking until now. Rudelius et al analyzed the Akt pathway in 12 cases of aggressive blastoid MCL as well as 16 typical MCLs and found that all of the aggressive blastoid MCLs exhibited constitutive phosphorylation of Akt, an indication that Akt was activated. The typical MCLs exhibited constitutive Akt phosphorylation at a much lower frequency and, often, to a lower degree. Akt promotes cell growth, cell survival, and tumorigenesis by phosphorylating multiple proteins.4 Akt-dependent phosphorylation inhibits FKHRL-1, a transcription factor that induces the expression of cell cycle arrest proteins; p27kip1, a negative regulator of cell cycle progression; and BAD, a proapoptotic member of the Bcl-2 family. As well, Akt enhances cell survival by promoting activation of NF-κB, a transcription factor that turns on antiapoptotic genes. Akt also increases protein translation, and hence cell growth, by promoting the phosphorylation of mammalian target of rapamycin (mTOR) and p70 S6 kinase and favors tumorigenesis by phosphorylating MDM-2, a negative regulator of the p53 tumor suppressor. Consistent with these well-known effects of Akt signaling, Rudelius and colleagues show that constitutive phosphorylation of Akt in the blastoid MCLs is accompanied by constitutive phosphorylation of FKHRL-1, p27kip1, mTOR, p70 S6 kinase, and MDM-2. The authors also show that treating blastoid MCL cells with chemical inhibitors of either Akt or its upstream activator PI3K reduced the phosphorylation of all of these proteins in addition to reducing NF-κB activation in the MCL cells. Importantly, these effects correlated with increased apoptosis and decreased proliferation of the MCL cells. Thus, inhibiting the PI3K/Akt pathway could be an important tool for treating blastoid MCLs, particularly the subset of blastoid MCLs in which these authors show that enhanced activation of the PI3K/Akt pathway is accompanied by down-regulation of PTEN, a negative regulator of PI3K signaling. Indeed, an inhibitor of mTOR (a downstream target of Akt) has shown antitumor activity toward MCLs in phase 2 clinical trials.5 Although more needs to be learned about the alterations that drive the survival and proliferation of MCLs (eg, how the PI3K pathway regulates cyclin D1), this paper demonstrates that the paradigm shown in Figure 1 can lead to new insights that can hopefully be translated into new treatments. ▪
From tumor to treatment. Molecular profiling of tumors can lead to the identification of key signal transduction pathways that may be dysregulated in a specific type of tumor (steps 1-4). In vitro studies on tumor cells can validate that the signaling pathway is aberrantly activated or inactivated in such a way that it favors cell growth, survival, and proliferation (step 5). Such studies may reveal that these signaling pathways are altered most frequently and to the greatest extent in the more aggressive forms of the tumor. A critical in vitro test is whether drugs that modulate the pathway that is dysregulated in these tumors can inhibit the survival, growth, or metastasis of the cells (step 6). Encouraging in vitro results can pave the way for clinical trials and new treatments (steps 7-8). The eventual goal would be to test tumor biopsies for characteristic tumor signatures and, based on the results, initiate the appropriate treatment (dashed line). With regard to mantle cell lymphoma, the paper by Rudelius and colleagues in this issue of Blood makes significant advances in terms of steps 5 and 6. Parts of this figure reproduced by permission. Originals, © 2003 Massachusetts Medical Society1 and © 2001 The Rockefeller University Press.2
From tumor to treatment. Molecular profiling of tumors can lead to the identification of key signal transduction pathways that may be dysregulated in a specific type of tumor (steps 1-4). In vitro studies on tumor cells can validate that the signaling pathway is aberrantly activated or inactivated in such a way that it favors cell growth, survival, and proliferation (step 5). Such studies may reveal that these signaling pathways are altered most frequently and to the greatest extent in the more aggressive forms of the tumor. A critical in vitro test is whether drugs that modulate the pathway that is dysregulated in these tumors can inhibit the survival, growth, or metastasis of the cells (step 6). Encouraging in vitro results can pave the way for clinical trials and new treatments (steps 7-8). The eventual goal would be to test tumor biopsies for characteristic tumor signatures and, based on the results, initiate the appropriate treatment (dashed line). With regard to mantle cell lymphoma, the paper by Rudelius and colleagues in this issue of Blood makes significant advances in terms of steps 5 and 6. Parts of this figure reproduced by permission. Originals, © 2003 Massachusetts Medical Society1 and © 2001 The Rockefeller University Press.2