The antithrombotic efficacy of lepirudin in patients with heparin-induced thrombocytopenia (HIT) is compromised by an increased risk for bleeding. A retrospective observational analysis in 181 patients (median age, 67 years) with confirmed HIT treated in routine practice with lepirudin was performed to identify predictive factors for thrombotic and bleeding complications. Lepirudin was administered at a mean (± SD) dose of 0.06 ± 0.04 mg/kg/h (compared with a recommended initial dose of 0.15 mg/kg/h). Mean activated partial thromboplastin time was greater than 1.5 times baseline value in 99.4% of patients. Median treatment duration was 7.7 days. Until discharge from the hospital, 13.8% and 20.4% of patients experienced a thrombotic or a major bleeding event, respectively. On multivariate analysis, mean lepirudin dose was not a significant predictive factor for thrombosis. In contrast, mean lepirudin dose greater than 0.07 mg/kg/h, long duration of lepirudin treatment, and moderate to severe renal impairment were significant positive factors for major bleeding. Overall, these results suggest that the recommended dose of lepirudin in patients with HIT is too high; the use of reduced doses may be safer with regard to bleeding risk and does not compromise antithrombotic efficacy.
Introduction
Heparin-induced thrombocytopenia (HIT) is a severe complication of heparin treatment that occurs at frequencies of 2.6% with unfractionated heparin and 0.2% with low-molecular-weight heparins.1 HIT is caused by heparin-dependent antibodies, generally directed toward the complex formed between heparin and platelet factor 4, which induce a prothrombotic state by activating platelets and endothelial cells.2,3 Treatment of HIT requires not only the immediate discontinuation of heparin therapy but also the use of alternative anticoagulants. Treatment options include danaparoid and direct thrombin inhibitors.4,5 Direct thrombin inhibitors are attractive because increased thrombin generation is believed to underlie the marked prothrombotic nature of HIT. Among direct thrombin inhibitors, lepirudin (Refludan; Pharmion, Cambridge, United Kingdom; Berlex Laboratories, Wayne, NJ), a recombinant hirudin, is approved worldwide for the treatment of patients with HIT, with or without thrombosis. Three prospective studies in patients with HIT demonstrated that lepirudin, administered according to its recommended dosage regimen, reduced the occurrence of new thrombotic events by more than 90%, but at the cost of increased bleeding risk.6-8 Thus, it is possible that this recommended dosage regimen may not be optimal in terms of antithrombotic benefit-to-bleeding risk ratio. To identify predictive factors for thrombotic and bleeding complications, we performed a retrospective observational study in 181 patients with HIT treated in routine practice with lepirudin, focusing in particular on the influence of the lepirudin dosage regimen on the occurrence of these events.
Patients, materials, and methods
This was a multicenter, retrospective, observational study conducted in France and Switzerland (Geneva) under the auspices of the French national group of clinical physicians and biologists working in the fields of hemostasis and thrombosis (Groupe d'Etude sur l'Hémostase et la Thrombose [GEHT]).
Patients
Data of all patients older than 16 years who had suspected or confirmed HIT and who were administered lepirudin between December 1997 and December 2004 were collected in 32 centers (see “Appendix”). HIT diagnosis was confirmed by an independent critical event committee on the basis of clinical or laboratory data.
Data collection
A number of complementary approaches were used to ensure the exhaustiveness of the identification of HIT patients treated with lepirudin during the study period. First, GEHT members were asked to report all patients meeting the inclusion criteria. Second, pharmacists of public or private hospitals to which lepirudin had been sold by the various manufacturers of this product (Schering, Aventis, and Pharmion) were contacted by mail and telephone to determine the use of each lepirudin batch supplied. Clinical departments to which lepirudin had been delivered were contacted to identify HIT patients in whom this treatment had been used. Finally, all French drug monitoring centers were contacted to obtain the list of patients who had experienced HIT. In each center with potentially eligible patients, a clinical research assistant collected the data using a standardized case report form. All data were subsequently centralized by the center responsible for data analysis (Centre d'Investigation Clinique, Hôpital Bellevue, Saint-Etienne, France).
Study outcomes
Primary study outcomes were the occurrence of thrombotic and bleeding events while patients were treated with lepirudin. Thrombotic episodes (including amputations) were confirmed on the basis of clinical findings or imaging data (phlebography, duplex ultrasonography, lung imaging, pulmonary angiography, or computed tomography). Major bleeding was defined as fatal bleeding, bleeding in a critical organ (retroperitoneal, intracranial, or intraocular), deep hematoma, or overt bleeding associated with a 20 g/L or greater decrease in hemoglobin level or leading to the transfusion of at least 2 U packed red blood cells. The observation period lasted from the beginning of lepirudin treatment until hospital discharge, which always occurred after the cessation of lepirudin treatment. A central, independent critical event committee adjudicated all efficacy and safety outcomes.
Statistical analysis
Quantitative data are reported as the mean, standard deviation (SD), median, first quartile (Q1), and third quartile (Q3). Qualitative data are reported as numbers and percentages. Univariate and multivariate analyses (logistic regression model) were used to determine the factors associated with the occurrence of thrombotic and bleeding events in patients treated with lepirudin. Stepwise modeling was performed to screen potential variables for inclusion in the final model. P values of no more than .20 were required for a variable to be included from each model (univariate analysis). P values of no more than .05 were taken as the threshold for statistical significance in the final model. Potential variables for these analyses were selected on the basis of their clinical plausibility. Data were processed and analyzed using SAS-Windows software (version 8.2).
Results
Patients
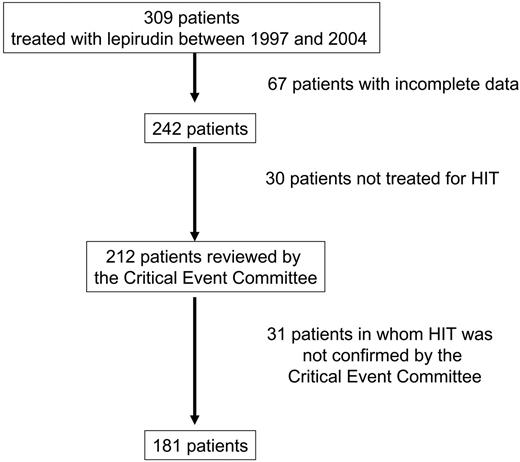
Data of 309 patients treated with lepirudin, regardless of the indication, were collected between December 1997 and December 2004 (Figure 1). Among 212 patients with complete data treated with lepirudin for suspected HIT, the critical event committee confirmed the use of lepirudin for documented HIT in 181 patients, who became the study population (Table 1). The median age of the population was 67 years; 22.1% of the patients were at least 75 years old. Heparin had been administered within the previous 3 months in 26.0% of the patients. Approximately 10% of the patients reported a history of venous thrombosis, and 10% reported a history of arterial thrombosis. Renal impairment (creatinine clearance less than 60 mL/min) was observed in 44.7% of the patients.
Heparin-induced thrombocytopenia
Most (81.2%) patients with HIT received unfractionated heparin alone or associated with a low-molecular-weight heparin at the time of the event (Table 2). Heparin was given at therapeutic doses (targeting activated partial thromboplastin time [aPTT] prolongation of 1.5-2.5 times baseline value) in 63.5% of the patients. The main indications for heparin therapy were management of cardiac and vascular diseases (37.6%) and venous thromboembolism treatment (22.7%).
HIT occurred after a median of 10.7 days of heparin treatment (Table 3). Median platelet count decreased from 217 × 109/L before heparin therapy to 55 × 109/L just before heparin cessation. The decrease in platelet count was greater than 30% of baseline values in 98.9% (178 of 180) of the patients; blood platelet count was below 100 × 109/L in 88.4% (160 of 181) of patients. Disseminated intravascular coagulation was associated with HIT in 4.4% (8 of 181) of patients. Diagnosis of HIT was biologically confirmed in 89.5% of patients, primarily by enzyme-linked immunosorbent assay (ELISA) to detect antibodies against heparin-platelet factor 4 complexes. Approximately half the patients (49.2%) experienced a thrombotic episode, whereas 6.1% had a major bleeding event. Approximately one third of the patients (32.6%; 59 patients) were first treated with danaparoid after heparin discontinuation. This treatment was replaced by lepirudin after a median (Q1-Q3) of 3.3 days (range, 1.0-6.5 days) because the blood platelet count remained low (61.0%; 36 of 59 patients), the patient did not show any clinical improvement (44.1%; 26 of 59 patients), or danaparoid cross-reacted with the patient's heparin-dependent antibodies (15.3%; 9 of 59 patients).
Treatment with lepirudin
Fifty-nine percent (105 of 178) of the patients received a bolus dose of lepirudin at treatment initiation. Mean (± SD) lepirudin dose during the entire treatment period was 0.06 ± 0.04 mg/kg/h; it ranged from less than 0.04 mg/kg/h to 0.15 mg/kg/h in 99.4% of patients (Table 4). The dose was significantly (P = .02) higher in patients with a thrombotic event (0.07 ± 0.04 mg/kg/h) than in asymptomatic patients (0.05 ± 0.05 mg/kg/h). Of 31 patients with normal renal function and isolated HIT, only 4 patients received 0.15 mg/kg/h lepirudin. Mean dose was significantly (P < .001) lower in patients with creatinine clearance lower than 60 mL/min (0.04 ± 0.03 mg/kg/h) than in patients with creatinine clearance greater than 60 mL/min (0.07 ± 0.04 mg/kg/h). Mean lepirudin dose significantly (P < .001) decreased between 1997 and 2001. This decrease was progressive. For example, it was 0.09 ± 0.04 mg/kg/h in 1997, 0.06 ± 0.03 mg/kg/h in 2001, and 0.04 ± 0.02 mg/kg/h in 2004. Mean aPTT was more than 1.5 times baseline value in 99.4% of patients. Median treatment duration was 7.7 days. Vitamin K antagonists were subsequently initiated in 84.2% of lepirudin-treated patients.
During the study period, 13.8% of patients experienced a thrombotic episode (Table 5). Median (Q1-Q3) time between the initiation of lepirudin therapy and thrombosis was 4 days (range, 2-6 days). Mean dose of lepirudin was 0.08 ± 0.04 mg/kg/h, and median (Q1-Q3) aPTT prolongation was 2.9 (range, 1.9-6.4) times baseline value.
During the study period, 37 patients (20.4%) had a major bleed. In 14 patients, the bleeds occurred during bridging between lepirudin and vitamin K antagonist treatments. Major bleeds were fatal in 7 patients (massive hemoptysis in 3 patients, intra-abdominal hemorrhage in 2 patients, intrathoracic hemorrhage in 1 patient, and retroperitoneal hemorrhage in 1 patient). Among these 7 patients, 2 patients had disseminated intravascular coagulation, 1 received aspirin 6 days earlier, and 4 recently underwent major surgery. All but 1 patient had renal insufficiency, and a bolus of lepirudin was administered to 3 patients. Mean dose of lepirudin was lower than 0.04 mg/kg/h in 2 patients and between 0.07 and 0.15 mg/kg/h in 4 patients; 1 patient received a single bolus of 0.2 mg/kg. The aPTT was prolonged 2.5 times above baseline value in 4 patients.
By the end of the study period, 46 patients (25.4%) had died. Six deaths were related to thrombotic events (Table 5) and 7 to major bleeds. Among the 33 other patients, HIT was considered a major factor in the death of 14 patients and a contributing factor in the death of 19 patients.
Predictive factors for thrombosis or major bleeding in HIT patients treated with lepirudin
On multivariate analysis, only the administration of therapeutic doses of heparin was a significant positive predictive factor for thrombosis in HIT patients treated with lepirudin (Tables 6 and 7). Moderate to severe renal impairment, long duration of lepirudin treatment, and mean lepirudin dose greater than 0.07 mg/kg/h were significant positive factors for major bleeding.
Discussion
The first main finding of this observational study was that, in routine practice, the mean lepirudin dose administered to patients with HIT was lower than the dose recommended in the drug labeling for this indication (administration of a 0.4 mg/kg bolus dose followed by a continuous intravenous dose of 0.15 mg/kg/h, adjusted to maintain an aPTT 1.5-2.5 times baseline value). Nevertheless, the mean aPTT was greater than 1.5 times baseline value in almost all patients (98.8%). This result is not entirely explained by the presence of renal impairment in approximately half the patients (patients in whom the recommended lepirudin dose was lower9 ) because the mean lepirudin dose was also low (0.07 mg/kg/h) in patients with normal creatinine clearance. Moreover, the dose administered steadily diminished between 1997 and 2004, indicating that prescribing practices changed as familiarity with lepirudin treatment increased. Recent studies have also reported that the actual doses administered to HIT patients were lower than those recommended.8,10-12
The second main finding was that, within this low dose range, mean lepirudin dose was nevertheless not an independent predictive factor for thrombotic complications. In contrast, it was an independent predictive factor for major bleeding (the higher the dose, the higher the bleeding risk). Overall, these results suggest that the dose of lepirudin recommended for HIT patients may be too high, and the use of reduced doses may be safer in terms of bleeding risk without compromising the antithrombotic efficacy. Our findings support the recent recommendation proposed by experts of the 2004 American College of Chest Physicians (ACCP) Consensus Conference to use lower lepirudin doses than those indicated in the drug labeling for patients with HIT.13
These data, reflecting the use of lepirudin in everyday practice, may be compared with those obtained in the most recent prospective controlled study in this patient population, the Heparin-Associated Thrombocytopenia (HAT)-3 study.8 In this study, the mean lepirudin doses were 0.11 mg/kg/h in HIT patients with thrombosis and 0.07 mg/kg/h in patients with asymptomatic HIT. Despite the fact that these mean doses were higher than those reported in our study (0.07 and 0.05 mg/kg/h, respectively), the rates of new thrombotic events and amputations after the initiation of lepirudin were comparable—11.5% (20 of 182) in HAT-3 and 13.8% in our study. In contrast, the risk for thrombosis in HIT patients in whom heparin therapy is discontinued without alternative anticoagulant treatment ranges from 19% to 52%.14 Thus, although our study was not primarily designed to demonstrate the efficacy of lepirudin, we observed that its result confirmed the value of this treatment, even at doses lower than those recommended. Furthermore, in our study, lepirudin efficacy might have been underestimated because several patients were first treated with danaparoid and then were switched to lepirudin (eg, because of danaparoid inefficacy), suggesting that HIT was particularly severe. Interestingly, in lepirudin-treated patients, previous use of therapeutic doses of unfractionated heparin was an independent predictive factor for thrombotic events. It is indeed possible that HIT patients treated with such heparin doses experienced more severe venous thromboembolic disease than patients receiving prophylactic doses of heparin.
The incidence of major bleeding observed in our study (20.4%) was in the upper range of the rates reported in a combined analysis of the 3 HAT lepirudin studies (17.6%; 95% CI, 14.0-21.7).8 However, the definition of major bleeding used in the present study was broader and included overt bleeding associated with a 20 g/L or greater decrease in hemoglobin level. In addition, the HAT studies excluded patients with overt signs of bleeding before the start of lepirudin treatment, whereas 6.1% of the patients analyzed in our study exhibited bleeding under heparin therapy. Moreover, the HAT-1 trial excluded patients with renal dysfunction.6 The fact that moderate or severe renal insufficiency was a predictive factor for major bleeding risk is not surprising because lepirudin is eliminated primarily through the kidneys.9 As previously observed,8,15 this result emphasizes the importance of careful dosing in patients with renal impairment. Interestingly, as in the recent HAT-3 study,8 age and sex were not significant risk factors for bleeding.
The limitations of this study were mostly related to its retrospective design, which involved the collection of data over a relatively long period of time during which the overall management of HIT patients might have changed. However, the conduct of prospective, randomized, controlled trials in this patient population is acknowledged to be problematic.14 We took care to ensure the exhaustiveness of the collection of all cases of lepirudin-treated HIT patients during the study period. Although the diagnosis of HIT was not biologically documented in 10.5% of the patients, it was confirmed in all patients on the basis of clinical or laboratory data by an independent critical event committee. It is also noteworthy that this is the first study in patients with HIT in whom thrombotic and bleeding events were validated by an independent critical event committee. Therefore, we believe that our data, obtained in a large number of patients, provide a valid picture of the routine management of HIT patients with lepirudin.
In conclusion, current dosing recommendations for lepirudin in patients with HIT may be too high. If this hypothesis is confirmed in prospective trials, the use of lower, but still effective, doses of lepirudin in HIT patients may improve the benefit-to-risk ratio of this drug for the management of this complex syndrome.
Appendix
Members of the Groupe d'Etude sur l'Hémostase et la Thrombose-Thrombopénie Induite par l'Héparine are as follows: steering committee—B. Tardy (Chair), Y. Gruel, T. Lecompte, P. Morange, B. Tardy-Poncet; writing committee—F. Boehlen, I. Elalamy, T. Lecompte, B. Tardy-Poncet, B. Tardy; critical event committee—P. Girard, M.-H. Horellou, P. Mismetti; Data Management and Statistical Analysis Center—E. Presles, S. Laporte (Unité de Pharmacologie Clinique, Hôpital Universitaire de Bellevue, Saint-Etienne, France); study monitors—F. Rancon, B. Deygas. Participating centers are as follows: Geneva University Hospital (F. Boehlen, P. De Moerloose, 30 patients); Etablissement de Transfusion Sanguine de Franche Comté, Besançon (E. Racadot, 16 patients); Hôpital Universitaire, Saint Etienne (B. Tardy-Poncet, J. Reynaud, 15 patients); Hôpital Universitaire, Antoine Béclère Clamart (M. Wolf, F. Parent F, 12 patients); Hôpital Foch, Suresnes (D. François, 9 patients); Institut Arnault Tzanck, St Laurent du Var (P. Camarasa, 7 patients); Hôpital Universitaire La Cavale Blanche, Brest (J.-F. Abgrall, M.-T. Blouch, 6 patients); Hôpital Universitaire Rangueil, Toulouse (B. Boneu, F. Nguyen, 6 patients); Centre Chirurgical Marie Lannelongue, Le Plessis Robinson (S. Doubine, 6 patients); Hôpital Universitaire Trousseau, Tours (Y. Gruel, C. Pouplard, 6 patients); Hôpital Universitaire du Bocage, Dijon (J.-L. Lorenzini, F. Dutrillaux, 6 patients); Hôpital Universitaire Européen Georges Pompidou, Paris (M. Alhenc-Gelas, 5 patients); Hôpital Universitaire Pitié-Salpétrière, Paris (A. Ankri, I. Martin-Toutain, 5 patients); Hôpital Universitaire, Lille (A. Bauters, 5 patients); Hôpital Universitaire Louis Pradel, Lyons (P. Ffrench, 5 patients), Hôpital Universitaire, Nancy (E. Demaistre, 4 patients); Hôpital Universitaire, Strasbourg (L. GruneBaum, J.-C. Thiranos, 4 patients); Hôpital Universitaire du Haut-Lévêque, Bordeaux (C. Mouton, 3 patients); Hôtel-Dieu, Paris (I. Elalamy, 3 patients); Hôpital Universitaire Gabriel Montpied, Clermont-Ferrand (M. Fouassier, 3 patients); Hôpital Universitaire de Bichat, Paris (M.G. Huisse, 3 patients), Hôpital Universitaire Henri Mondor, Creteil (M. Gouault-Heilmann, 3 patients); Centre Hospitalier, Angoulême (V. Lucke, 3 patients); Hôpital Universitaire Pontchaillou, Rennes (M. Laurent, 3 patients); Hôpital Universitaire Saint Eloi, Montpellier (C. Biron-Andreani, 2 patients); Centre Hospitalier, Guéret (A. Moret, 2 patients); Institut Mutualiste de Montsouris, Paris (J. Chasselut, 1 patient); Centre Hospitalier, Annecy (B. Blanc-Jouvan, 1 patient); Centre Hospitalier, Chambery (C. Fogliani, 1 patient); Hôpital Universitaire,Angers (C. Ternisien, 1 patient); Hôpital Universitaire, Amiens (C. Tribout, 1 patient); Hôpital Universitaire Hôtel-Dieu, Nantes (M. Trossaert, 1 patient); Hôpital Universitaire Edouard Herriot, Lyons (M.-C. Trzeciak, 1 patient); Hôpital Universitaire Necker, Paris (D. Lasne, 1 patient); Hôpital Universitaire, Caen (A. Le Querrec, 1 patient).
Prepublished online as Blood First Edition Paper, May 11, 2006; DOI 10.1182/blood-2006-02-001057.
A complete list of the members of the Groupe d'Etude sur l'Hémostase et la Thrombose-Thrombopénie Induite par l'Héparine (GEHT-TIH) appears in “Appendix.”
Supported in part by grants from Programme Hospitalier de Recherche Clinique (PHRC) of the Ministère Français de la Santé, and Pharmion France.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank M. Ollagnier (Drug Monitoring Center, Saint-Etienne, France) for helpful contributions. We also thank J.-Y. Darmon and Y. Cadroy (MediBridge Clinical Research, Vélizy, France) for editorial assistance.