Refractory anemia with ring sideroblasts (RARS) is a myelodysplastic syndrome characterized by an anemia in which at least 15% of bone marrow erythroblasts are ringed sideroblasts. A percentage of RARS patients (10%-20%) eventually present high platelet counts (> 500 × 109/L), which may be due to reactive thrombocytosis or secondary myeloproliferative disorder (MPD).1-3 The recently discovered V617F mutation of tyrosine-kinase JAK2 (JAK2-V617F),4 specific to MPD except chronic myeloid leukemia, is present in 98% of polycythemia vera and 70% of essential thrombocythemia (ET). By sequencing, the JAK2-V617F mutation has also been detected in 5% of myelodysplastic syndromes (4% of RARS).5 To investigate the presence of JAK2-V617F and MPD in patients with RARS and thrombocytosis (RARS-T), we used an allele-specific, sensitive quantitative PCR recently developed that detects 0.5% of JAK2-V617F.6
Sixteen patients diagnosed with RARS over a period of 20 years (1986-2005) and who subsequently presented with thrombocytosis were examined. Genomic DNA was prepared from blood granulocytes (2 patients) or stained bone marrow smears (14 patients) collected at the time of thrombocytosis. Quantitative PCRs were performed with forward primers 5′-GCGCGGTTTTAAATTATGGAGTATGTG-3′ (wild-type JAK2) and 5′-GCGCGGTTTTAAATTATGGAGTATGTT-3′ (JAK2-V617F), reverse primer 5′-GCGGTGATCCTGAAACTGAATTTTC-3′ and 6-FAM probe 5′-TGGAGACGAGAGTAAGTAAAACTACAGGCT-3′ as described elsewhere.6 Wild-type JAK2 was amplified successfully for the 16 patients; JAK2-V617F was amplified for 5 patients. As observed in ET,6 the level of expression of JAK2-V617F was less than 40% of total JAK2 (median, 16%; range, 4%-35%). For 1 patient, JAK2-V617F was also amplified from a bone marrow smear performed 5 years earlier, at a time when platelet counts were normal.
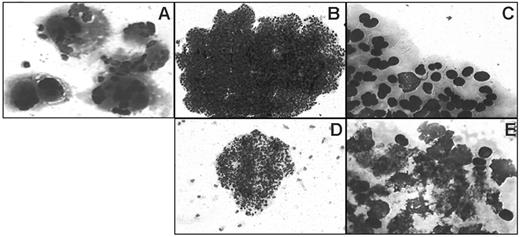
A bone marrow biopsy was performed for 10 patients: histology was in favor of ET for 7 patients (4 positive for JAK2-V617F, 3 without the mutation) (Table 1); for the fifth patient carrying the mutation, no biopsy was performed. Altogether, 8 of the 16 patients with RARS-T could be diagnosed with ET either according to the World Health Organization criteria or because they carried the JAK2-V617F mutation, or both. Patients with RARS-T diagnosed with ET differed from other patients by a lower percentage of sideroblasts and higher platelet counts (Table 1). In this limited series, expression of JAK2-V617F did not affect blood cell counts and was not associated with myelofibrosis,7 observed for only 1 patient (negative for the mutation). Last, the collagen assay of endogenous colony formation, which allows easy identification of megakaryocytic colonies (Figure 1) and is positive in 85% of ET,8 including those negative for JAK2-V617F,6 was performed for 4 of the 5 JAK2-V617F-positive patients. Endogenous colony assays were negative for the 4 patients; moreover, cell death was common in erythroid colonies grown in the presence of cytokines, which is not observed in ET but typical of myelodysplastic syndromes (Figure 1).
In summary, 50% of RARS patients with high platelet counts presented ET features; 31% carried the JAK2-V617F mutation. ET secondary to RARS differs from primary ET by the absence of formation of endogenous colonies.
Typical colonies observed in ET and in RARS with ET features. Colonies are shown after May-Grünwald-Giemsa staining of collagen gels and are typical of ET (A-C) or of RARS with ET features (D-E). (A) Endogenous megakaryocytic colony, grown without serum or cytokine. (B,D) Erythroid colonies grown in the presence of cytokines; note the small size of the RARS colony (D). (C) Detail of erythroblasts of the colony shown in panel B. (E) Detail of erythroblasts of the colony shown in panel D; note extensive cell death. Images were obtained using a Leica microscope (Leica Microsystems, Wetzlar, Germany) equipped with a 10×/0.80 numeric aperture (NA) (A,B,D) or a 100×/0.80 NA (C,E) objective. Images were acquired using a Sony Power HAD DXC-950P camera (Sony, Tokyo, Japan) and Tribun ICS/Thunder 17 acquisition software. Images were processed using Microsoft PowerPoint 2000 (Microsoft, Redmond, WA).
Typical colonies observed in ET and in RARS with ET features. Colonies are shown after May-Grünwald-Giemsa staining of collagen gels and are typical of ET (A-C) or of RARS with ET features (D-E). (A) Endogenous megakaryocytic colony, grown without serum or cytokine. (B,D) Erythroid colonies grown in the presence of cytokines; note the small size of the RARS colony (D). (C) Detail of erythroblasts of the colony shown in panel B. (E) Detail of erythroblasts of the colony shown in panel D; note extensive cell death. Images were obtained using a Leica microscope (Leica Microsystems, Wetzlar, Germany) equipped with a 10×/0.80 numeric aperture (NA) (A,B,D) or a 100×/0.80 NA (C,E) objective. Images were acquired using a Sony Power HAD DXC-950P camera (Sony, Tokyo, Japan) and Tribun ICS/Thunder 17 acquisition software. Images were processed using Microsoft PowerPoint 2000 (Microsoft, Redmond, WA).
Supported by a grant from the Délégation Régionale à la Recherche Clinique of the Région Pays de Loire and the CHU de Nantes.