Comment on Rolny et al, page 1877
In addition to its well-established roles in maintaining vessel stability by recruiting pericytes to capillaries and promoting smooth muscle cell development in vessels, PDGFRβ may be expressed on early hematopoietic/endothelial precursors and play a role in regulating vascular/hematopoietic development. Coinhibition of PDGFRβ activity may represent a unique and effective approach to enhancing therapeutic efficacy of antiangiogenic as well as conventional chemo- or radiotherapy.
Platelet-derived growth factor (PDGF) receptor β (PDGFRβ) has been shown to be primarily responsible for pericyte recruitment to capillaries and development of smooth muscle cells in vessels and mesangial cells in the kidney. Knockout of PDGFRβ or its ligand, PDGF-BB, in mice results in organ-specific hemorrhages and edema, due mainly to defection in pericyte coverage on newly formed vessels.1,2 Recently, PDGFRs have also been detected on lymphatic vessels. PDGF-BB not only promotes lymphatic endothelial cell motility in vitro, but it also potently induces intratumoral lymphangiogenesis in vivo, leading to enhanced tumor lymphatic metastasis.3 Further, it has been shown that vascular endothelial growth factor receptor 2 (VEGFR2)–positive embryonic stem cells, which form endothelial cells when exposed to VEGF, could differentiate into mural cells in the presence of PDGF-BB.4 Taken together, these findings suggest that PGDF/PDGFR signaling pathway plays important roles in modulating both angiogenic and lymphoangiogenic processes.FIG1
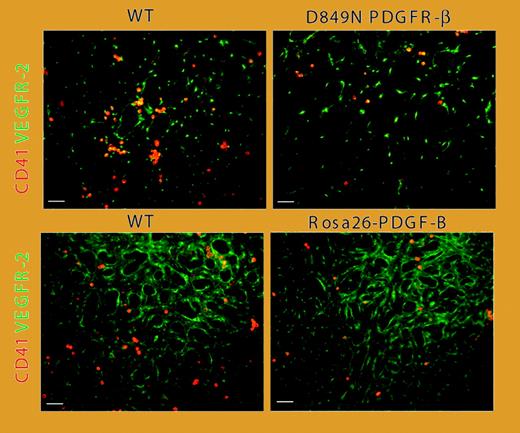
Reduced number of CD41-expressing hematopoietic cells and increased vascular remodeling in PDGF-BB/PDGFR-β–activated yolk sacs. See the complete figure in the article beginning on page 1877.
Reduced number of CD41-expressing hematopoietic cells and increased vascular remodeling in PDGF-BB/PDGFR-β–activated yolk sacs. See the complete figure in the article beginning on page 1877.
In this issue of Blood, Rolny and colleagues provide evidence suggesting that PDGFRβ is expressed on CD31+CD41+-VEGFR2+ early hematopoietic/endothelial precursors, or hemangioprecursors. Activation of PDGFRβ on these hemangioprecursors accelerated differentiation of endothelial cells, whereas differentiation of the hematopoietic lineage was suppressed. Yolk sacs derived from mice expressing a kinase-activating PDGFRβ mutant or from mice with ubiquitous PDGF-BB expression showed a decrease in the numbers of CD41+ early hematopoietic cells, along with increased differentiation of endothelial cells and vascular remodeling (see figure). Taken together, these results suggest that PDGFRβ expressed on early hematopoietic/endothelial precursors may play an important role in regulating vascular/hematopoietic development.
While the importance of PDGFβ in maintaining vascular stability is well established, its function in regulating early hematopoietic development by affecting the rates of differentiation of both endothelial and hematopoietic lineages is rather unexpected. Rafii and colleagues5 previously described the identification of 2 angiogenesis-relevant progenitor cell populations: CD133+CD34+c-Kit+VEGFR2+ (sca-1+c-Kit+LinnegVEGFR2+ in mouse) endothelial progenitor cells(EPCs) and CD31+CD34+ c-Kit+VEGFR1+ (sca-1+c-kit+Linneg-VEGFR1+ in mouse) hematopoietic stem and progenitor cells (HSCs and HPCs). Comobilization from bone marrow into circulation and corecruitment of HSCs/HPCs and EPCs by angiogenic stimuli may represent the major contributing factors to the initiation and sustenance of neovascularization under both physiologic and pathologic conditions, such as ischemia, wound healing, and cancer. While inhibition of individual vascular endothelial growth factor receptor 1 (VEGFR1) or VEGFR2 only partially delayed tumor growth in xenograft models, simultaneous neutralization of both receptors using antibody combination led to potent antiangiogenic response and tumor regression, suggesting that blockade of mobilization and recruitment of both HSCs/HPCs and EPCs are essential for optimal antiangiogenic/antitumor activity. Further, inhibition of VEGFR1 (but not VEGFR2) blocked HSC cell cycling, differentiation, and hematopoietic recovery after bone marrow suppression. In light of these observations, the nature of the PDGFRβ+ CD31+CD41+VEGFR2+ cell population described by Rolny and colleagues, its relevance or linkage to other previously described angiogenesis-relevant cell populations such as HSCs/HPCs and EPCs, and the physiologic significance of PDGFRβ expression on these cells deserve further careful study. In this study, the authors suggest that the PDGFRβ+ hemangioprecursors may represent a transient, heterogeneous hemangioblastic cell population. PDGF-BB, functioning as a switch toward endothelial cell development, may induce endothelial cell differentiation directly, by directly stimulating PDGFRβ expressed on the hemangioprecursors, or indirectly, by stimulating the production of other growth factors (such as VEGF) by the committed perivascular cells. As a consequence, the differentiation of hemangioprecursors into the hematopoietic lineage was negatively suppressed. Additional proof for the authors' hypothesis might be provided by future research examining whether PDGFRβ inhibition, for example, via selective PDGFRβ kinase inhibitors (such as imatinib mesylate) or neutralizing antibodies to PDGFRβ or PDGF-BB, could reverse the trend of differentiation between the endothelial and hematopoietic lineages in the yolk sacs that express the kinase-activating PDGFRβ mutant or with ubiquitous PDGF-BB expression. However, it is worth noting that, in addition to hemorrhages and defects in kidney glomeruli because of a lack of mesangial cells, mice with PDGFRβ or PDGF-BB knockout also manifest severe hematologic abnormalities, including thrombocytopenia, erythroblastosis, and severe anemia.1,2
With the success of bevacizumab, an anti-VEGF antibody, the antiangiogenesis approach is gradually being accepted as an effective and practical means of anticancer therapy. A number of strategies are being explored to directly target the endothelial cells by inhibiting their growth/survival mechanisms (for example, with the use of antibodies to VEGF and its receptors6 ). Recently, pericytes have been heralded as potential targets for vascular destruction. Since pericyte recruitment to coat nascent vessels is essential for the stabilization and further establishment of the vascular network, vessels lacking adequate pericyte coverage are more vulnerable to VEGF inhibition.7 Of the apparent approaches for negatively affecting pericyte coverage and function, perhaps the most logical and effective is to block the PDGFβ signaling pathway in these cells. However, it is rather surprising that only very limited antiangiogenic/antitumor benefits have been achieved so far when PDGFRβ antagonists have been tested as single agents. On the other hand, PDGFRβ antagonists have been shown to significantly enhance the antitumor activity of a number of chemotherapeutic agents when used in combination.8 The enhanced antitumor effect usually correlates well with increase in tumor uptake of the cytotoxic agents, most likely as a result of reduction of interstitial fluid pressure in tumor after treatment with PDGFRβ antagonists.8 A number of studies have demonstrated that PDGFRβ antagonists (antipericytes) could also generate additive/synergistic antitumor activity when combined with anti-VEGFR2 agents (antiendothelial cells), such as an anti-VEGFR2 antibody (DC101; J. Shen and Z. Z., unpublished observations, 2006) and small molecule inhibitors to VEGFR2 kinase.9 Recently, Song and colleagues10 reported the identification of a subset of PDGFRβ+/ sca-1+ progenitor perivascular cells (PPCs) that could be recruited from bone marrow to perivascular sites in tumors. Through a paracrine mechanism involving growth factors produced by endothelial cells, including PDGF-BB, such precursors, like the previously described VEGFR2+ embryonic stem cells,4 could differentiate into mature pericytes and regulate vessel stability and survival in tumors. Specific inhibition of PDGFRβ signaling with a neutralizing antibody to the receptor eliminates PDGFRβ+ PPCs and mature pericytes around tumor vessels, leading to increased endothelial cell apoptosis.10 Taken together, these findings should lend support to the use of PDGFRβ antagonists in combination with other antitumor and/or antiangiogenic agents in the treatment of a broad range of human cancers. ▪