Comment on Lee et al, page 2064
Targeted disruption of ICAM-4 in mice alters the integrity of erythroblast islands, the microenvironmental niches in marrow where erythroid precursors mature while attached to central macrophage cells.
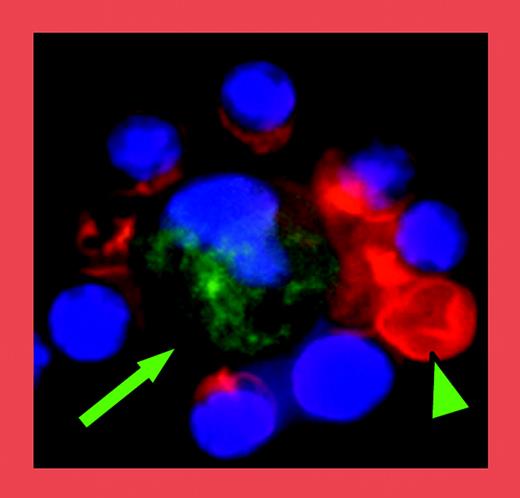
Half a century ago, researchers recognized that all maturing erythroid precursors are physically associated with macrophage cells.1 Intact “erythroblast islands” consisting of a macrophage cell with a ring of attached immature nucleated red cells can be isolated from the bone marrow following gentle enzymatic treatment (see figure). Erythroblast islands had remained unidentified because they break up when marrow cells are routinely smeared onto microscope slides. Several adhesion molecules that mediate the physical interactions among erythroblasts or between erythroblasts and macrophage cells have been described, including erythroblast macrophage protein (Emp), α4β1 integrin, and VCAM-1 molecules.2,3
In this issue of Blood, Lee and colleagues describe an important role for intercellular adhesion molecule-4 (ICAM-4) in erythroblast island integrity. ICAM-4, a member of the immunoglobulin superfamily, is expressed by erythroblasts and interacts with αv integrin, which is expressed by macrophage cells. The authors developed a novel assay to quantify erythroblast island numbers and determined that mice lacking ICAM-4 have significantly fewer islands that can be isolated from marrow of normal mice. Erythroid and macrophage cells can reconstitute islands when single-cell preparations of marrow cells are incubated in vitro. Marrow cells lacking ICAM-4 have a markedly reduced ability to reconstitute erythroblast islands, compared with marrow cells from wild-type mice. Furthermore, peptides capable of blocking ICAM-4 interactions with αv integrin also reduce the number of reconstituted erythroblast islands in a dose-dependent manner. These complementary findings indicate that ICAM-4 plays a significant role in the ability of erythroblasts to physically associate with macrophage cells in the marrow.
Interestingly, these defects in erythroblast islands' integrity do not lead to alterations in steady-state erythropoiesis, since the adult ICAM-4–null mouse has a normal red cell mass and reticulocyte count. It would be interesting to know if the erythroid response to acute anemia is impaired by the lack of ICAM-4. Furthermore, the role of ICAM-4 in the fetus, which rapidly synthesizes erythrocytes within erythroblastic islands in the liver, is worthy of future study.
These findings in ICAM-4–null mice raise questions regarding the role of erythroblast islands in terminal erythroid differentiation. It has long been postulated, although never proven, that central macrophage cells nurse erythroblasts by supplying them with nutrients, such as iron, and cytokines, such as erythropoietin. The close physical interaction of maturing erythroblasts in islands may also facilitate the regulation of red cell numbers.4 Finally, it is well established that macrophage cells ingest the nuclei left behind by reticulocytes as they leave their island home and enter the turbulent world of the bloodstream.5 Ultimately, how macrophage cells specifically support erythroid cellFIG1 maturation remains to be clarified. This report provides a new in vivo model and a novel in vitro approach for further investigation of this important question. ▪
Immunofluorescent analysis of an erythroblast island consisting of a central macrophage (green; arrow) surrounded by erythroid cells (red), one of which has enucleated (arrowhead). See the complete figure in the article beginning on page 2064.
Immunofluorescent analysis of an erythroblast island consisting of a central macrophage (green; arrow) surrounded by erythroid cells (red), one of which has enucleated (arrowhead). See the complete figure in the article beginning on page 2064.