Comment on Shang et al, page 2207
In this issue of Blood, Shang and colleagues demonstrate that endothelial cells of different vascular beds synthesize and release ADAMTS13, the metalloprotease that cleaves ultra-large von Willebrand factor (ULVWF). The release is targeted to the luminal side of endothelial cells by the association of the C-terminal CUB domains with membrane lipid rafts.
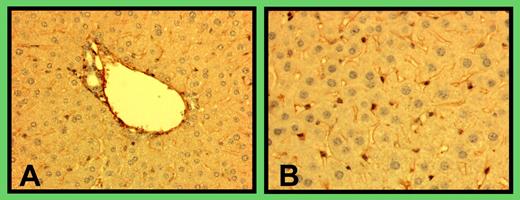
The current study, together with another report,1 has greatly expanded cellular sources of the metalloprotease, which was originally believed to be primarily synthesized in hepatic stellate cells.2,3 The study is consistent with the demonstrated presence of ADAMTS13 mRNA in endothelial cells4 and, more importantly, puts the metalloprotease where its substrate ULVWF is synthesized, released, and likely cleaved. Although the data are generated in cell culture, endothelial cells are likely to synthesize the metalloprotease in vivo. Through immunohistochemistry, we have found that vascular endothelial cells, as well as stellate cells, in mouse liver are extensively stained for ADAMTS13 (see figure).FIG1
Immunohistologic staining of mouse liver for ADAMTS13. Liver was dissected from C57BL/6J mice, rinsed with PBS, fixed with 10% formalin, and sectioned at a thickness of 10 μm. The tissue section was stained with a goat anti–human ADAMTS13 (5 μg/mL; Bethyl Laboratory, Houston, TX) followed by an HRP-conjugated rabbit anti–goat IgG. The figure is a representation of immunohistologic staining of liver tissues from 5 mice. The lefthand panel depicts vascular staining, and the righthand panel, stellate cell staining.
Immunohistologic staining of mouse liver for ADAMTS13. Liver was dissected from C57BL/6J mice, rinsed with PBS, fixed with 10% formalin, and sectioned at a thickness of 10 μm. The tissue section was stained with a goat anti–human ADAMTS13 (5 μg/mL; Bethyl Laboratory, Houston, TX) followed by an HRP-conjugated rabbit anti–goat IgG. The figure is a representation of immunohistologic staining of liver tissues from 5 mice. The lefthand panel depicts vascular staining, and the righthand panel, stellate cell staining.
The endothelial cell–derived ADAMTS13 is shown to be constitutively released, not colocalized with ULVWF in Weibel-Palade bodies. However, it is not clear as to whether the newly synthesized ADAMTS13 is also targeted to other storage granules. The question is an interesting one because the study by Shang et al also demonstrates that the CUB domains in the C-terminus of ADAMTS13 associate with membrane lipid rafts of endothelial cells. Furthermore, in transfected cells of endothelial and epithelial lineages, this association directs the release of recombinant ADAMTS13 predominantly to the luminal side of endothelial cells. Such a polarity is lost or reversed when the CUB domains are deleted. The results logically explain why some mutations in the CUB domains significantly reduce the amounts of recombinants released to the culture medium, even though synthesis of these mutants appears to be normal. The association with membrane lipid rafts, which serve as a platform to assemble and convey intracellular signals, also raises the possibility that ADAMTS13 release is regulated by intracellular signals. Several interesting questions emerge if this is, indeed, the case. First, is release of ADAMTS13 from endothelial cells inducible? Second, if yes, what are the agonists and do they also induce ULVWF release? On one hand, if the same signals also induce UL-VWF release, the endothelial cell–derived ADAMTS13 could conveniently serve as a safeguard against a sudden surge of ULVWF release commonly found in conditions such as systemic inflammation. A similar mechanism has been implicated in platelets, another source of ULVWF.5 Upon activation, ADAMTS13 on the platelet surface increases significantly through intracellular release and capturing from plasma. On the other hand, signals that induce ULVWF release may be inhibitory to ADAMTS13 secretion. This may at least partly explain why ADAMTS13 activity is transiently reduced in healthy individuals who received bolus injection of either desmopressin or lipopolysaccharide, both of which induce acute release of ULVWF. In light of these possibilities, the current study provokes new thinking as to how ADAMTS13 synthesis and release are regulated in vivo, an area that has so far been largely unknown. ▪