Abstract
An acquired V617F JAK2 mutation occurs in patients with polycythemia vera (PV) or essential thrombocythemia (ET). In a proportion of V617F-positive patients, mitotic recombination produces mutation-homozygous cells that come to predominate with time. However, the prevalence of homozygosity is unclear, as previous reports studied mixed populations of wild-type, V617F-heterozygous, and V617F-homozygous mutant cells. We therefore analyzed 1766 individual hematopoietic colonies from 34 patients with PV or ET in whom granulocyte sequencing demonstrated that the mutant peak did not predominate. V617F-positive erythroid burst-forming units (BFU-Es) were more frequent in patients with PV compared with patients with ET (P = .022) and, strikingly, V617F-homozygous BFU-Es were detected in all 17 patients with PV, but in none of the patients with ET (P < .001). Moreover, mutation-homozygous cells were present in 2 patients with ET after polycythemic transformation. These results demonstrate that V617F-homozygous erythroid progenitors are present in most patients with PV but occur rarely in those with ET.
Introduction
An acquired V617F mutation in the tyrosine kinase JAK2 occurs in most patients with polycythemia vera (PV) and in half of those with essential thrombocythemia (ET) or idiopathic myelofibrosis.1-5 This mutation increases JAK2 kinase activity,2-5 confers cytokine independence on cell lines,2-5 is required for erythropoietin (Epo)–independent erythroid progenitor growth in affected patients,1,3 and produces erythrocytosis in a retroviral bone marrow transplantation model.3 JAK2 mutation status divides patients with ET into 2 distinct subgroups, with V617F-positive patients resembling a forme fruste of PV.6 These data have led to the suggestion that V617F-positive ET and PV form a disease continuum, with the extent of erythrocytosis influenced by a combination of genetic and physiologic modifiers.6
Homozygosity for the V617F mutation occurs in 30% of patients with PV,1-5,7 but is rare in patients with ET, suggesting that it may favor a polycythemic phenotype. These and other studies8-10 have inferred the presence of V617F homozygosity from sequence analysis of leukocyte DNA showing predominance of the mutant peak. However, patients with PV or ET in whom the mutant peak does not predominate may nonetheless harbor a subpopulation of V617F-homozygous cells. To investigate this possibility, we assessed the JAK2 mutation status of individual hematopoietic colonies from V617F-positive patients with PV or ET.
Study design
Mutation screening
Studies were approved by the Addenbrooke's NHS Trust Local Research Ethics Committee and the Multi-Regional Ethics Committee (MREC), United Kingdom. All patients gave verbal and written informed consent, and research was carried out according to the principles of the Declaration of Helsinki. Patient diagnoses were according to the modified Polycythemia Vera Study Group (PVSG) criteria for PV11 and ET.12 The clinical and biologic features of all patients are listed in Table S1 (available at the Blood website; see the Supplemental Materials link at the top of the online article). Patient white blood cell (WBC) preparation and JAK2 sequence analysis were performed as described.1 Sequence patterns were scored as V617F heterozygous if the mutant peak was 50% or less of total peak height. Peripheral blood erythroid burst-forming units (BFU-Es), Epo-independent erythroid colonies (EECs), and granulocyte-macrophage colony-forming units (CFU-GMs) were genotyped by BsaXI digestion.1,13 BsaXI digestion of JAK2 exon 14 was confirmed by digestion of a control (stem-cell leukemia [SCL]) fragment and/or by sequencing of colony DNA in all 700 colonies so analyzed.
Statistical methods
Frequencies of V617F-positive and V617F-homozygous colonies in patients with ET and PV were compared using an unpaired Student t test. Frequencies of V617F-homozygous colonies were compared in V617F-positive ET patients whose disease had not transformed and those whose had transformed to PV using the Fisher exact test. To assess the relationship between clinical/laboratory characteristics and the proportion of either V617F-homozygous BFU-Es or V617F-positive BFU-Es, logistic regression models were fitted, and significance tested using likelihood ratio tests.
Results and discussion
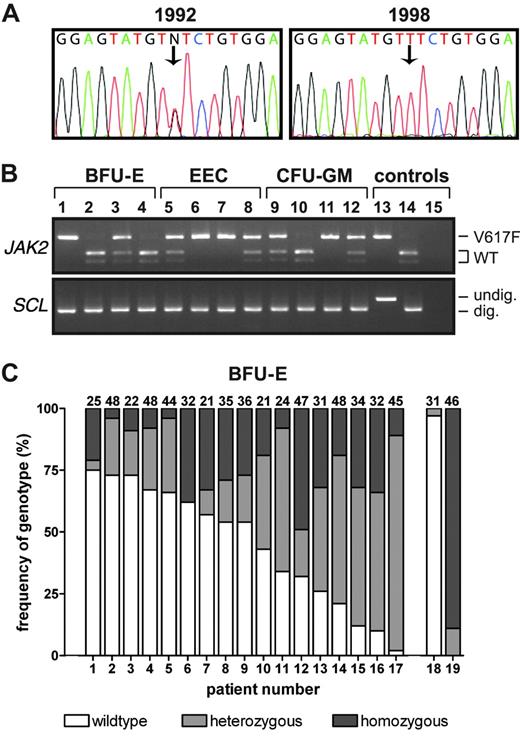
We identified 5 patients with PV and a V617F-homozygous sequence pattern in their granulocytes (mutant peak > 50% total peak height) for whom an earlier granulocyte DNA sample was available. In 3 patients, the earlier samples (taken 2, 5, or 6 years previously) had a heterozygous pattern (Figure 1A). These results demonstrate that V617F-homozygous clones may predominate with time, and are consistent with the concept that patients with an apparently V617F-heterozygous sequence pattern may harbor a subpopulation of V617F-homozygous cells.
Frequency of V617F homozygosity in patients with PV. (A) JAK2 sequence analysis of granulocyte DNA samples from a patient with PV. Note the V617F-heterozygous sequence pattern in a sample from 1992, and a V617F-homozygous pattern in a sample from 1998. (B) BsaXI digestion of JAK2 exon 14 and SCL intron 1 was used to genotype DNA from BFU-Es, EECs and CFU-GMs cultured from PV patient 13. Lanes 1-4 indicate BFU-Es; lanes 5-8, EECs; lanes 9-12, CFU-GMs; lane 13, undigested granulocyte DNA from a V617F-negative control; lane 14, BsaXI-digested granulocyte DNA from a V617F-negative control; and lane 15, water. V617F-homozygous colonies are seen in lanes 1, 6, 7, and 11; heterozygous colonies are seen in lanes 3, 5, 8, 9, and 12. (C) Frequencies of wild-type, V617F-heterozygous, and V617F-homozygous peripheral blood BFU-Es in 17 PV patients with a heterozygous JAK2 granulocyte sequence trace were determined using PCR and BsaXI digestion. The absolute number of colonies genotyped from each patient is at the top of each column. Column 18 shows a V617F-positive PV patient with a wild-type sequence trace; column 19, a PV patient with a V617F-homozygous sequence trace.
Frequency of V617F homozygosity in patients with PV. (A) JAK2 sequence analysis of granulocyte DNA samples from a patient with PV. Note the V617F-heterozygous sequence pattern in a sample from 1992, and a V617F-homozygous pattern in a sample from 1998. (B) BsaXI digestion of JAK2 exon 14 and SCL intron 1 was used to genotype DNA from BFU-Es, EECs and CFU-GMs cultured from PV patient 13. Lanes 1-4 indicate BFU-Es; lanes 5-8, EECs; lanes 9-12, CFU-GMs; lane 13, undigested granulocyte DNA from a V617F-negative control; lane 14, BsaXI-digested granulocyte DNA from a V617F-negative control; and lane 15, water. V617F-homozygous colonies are seen in lanes 1, 6, 7, and 11; heterozygous colonies are seen in lanes 3, 5, 8, 9, and 12. (C) Frequencies of wild-type, V617F-heterozygous, and V617F-homozygous peripheral blood BFU-Es in 17 PV patients with a heterozygous JAK2 granulocyte sequence trace were determined using PCR and BsaXI digestion. The absolute number of colonies genotyped from each patient is at the top of each column. Column 18 shows a V617F-positive PV patient with a wild-type sequence trace; column 19, a PV patient with a V617F-homozygous sequence trace.
We therefore analyzed 1082 hematopoietic colonies from 17 patients with PV who had a heterozygous sequence pattern recorded in their peripheral blood granulocytes in the preceding 6 months. These were assessed by BsaXI digestion,1,6,13 which distinguishes between wild-type, V617F-heterozygous, and V617F-homozygous genotypes (Figure 1B). As expected, BFU-Es from a patient with a homozygous granulocyte sequence pattern were predominantly V617F homozygous (Figure 1C; patient 19). However, V617F-homozygous BFU-Es were also present in all patients with a heterozygous granulocyte sequence (patients 1-17). To exclude the possibility that the BsaXI site was destroyed by mutations other than the 1849G>T (V617F) mutation, exon 14 was sequenced in colonies with a restriction pattern consistent with a V617F-homozygous genotype. In 48 colonies from 8 patients with PV, the 1849G>T mutation was detected in both JAK2 alleles. The frequencies of V617F-homozygous or total V617F-positive BFU-Es did not correlate significantly with age, sex, disease duration, or treatment, or with hemoglobin levels, WBC counts, or platelet counts either at diagnosis or the time of analysis (P > .5 for all variables). All patients analyzed within 3 months of diagnosis had V617F-homozygous BFU-Es (Figure 1C; patients 2-4, 9, and 11), suggesting that homozygosity occurs at an early stage of the clinically overt disease. As the V617F mutation is detectable as a homozygous or heterozygous granulocyte sequence trace in 80% of patients with PV,1-5 our demonstration that patients with PV who had a heterozygous sequence trace do harbor V617F-homozygous progenitors suggests that these cells are present in nearly all patients with PV. However, a few patients have a normal granulocyte JAK2 sequence but are V617F positive by polymerase chain reaction (PCR)–based methods.1 In one such patient, 3% of BFU-Es were V617F positive (Figure 1C; patient 18); the frequency of V617F-homozygous colonies may have therefore been below the level of detection, although it is possible that occasional patients with PV lack V617F-homozygous cells.
All 316 EECs from 15 of the patients with PV who had a V617F-heterozygous granulocyte sequence pattern had at least 1 mutant JAK2 allele, in agreement with previous observations.1 There was no consistent relationship between the proportion of mutation-positive colonies that were homozygous when cultured in the presence or absence of saturating Epo concentrations (116 of 289 vs 130 of 270 colonies, P = .4; Figure S1A). CFU-GM colonies (n = 96) from 6 patients with PV were also analyzed (Figure S1B). Mutation-homozygous colonies were identified in 5 patients, demonstrating that the mutational event responsible for V617F homozygosity occurs at or before the common myeloid progenitor stage. In each patient, the proportions of CFU-GMs and BFU-Es that were V617F positive were comparable, as were the proportions of CFU-GMs and BFU-Es that were V617F homozygous, suggesting that this mutation does not strongly bias commitment toward the erythroid lineage.
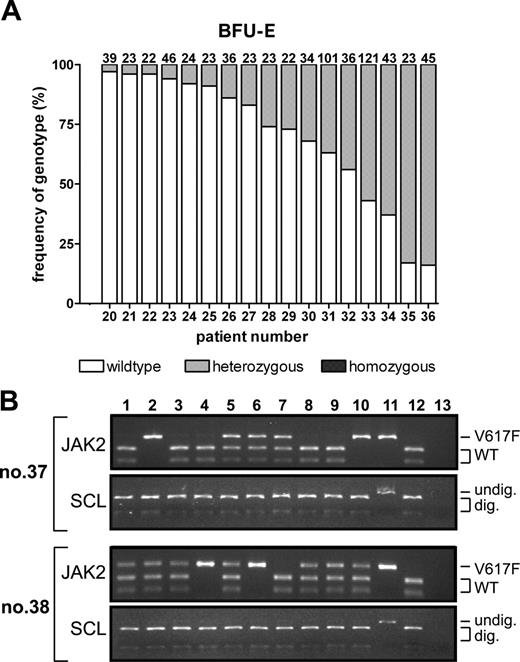
To investigate whether patients with ET also have a subpopulation of V617F-homozygous progenitors, we analyzed 684 BFU-Es from V617F-positive ET patients (Figure 2A; patients 20-36). V617F-heterozygous BFU-Es were identified in all patients. The proportion of V617F-positive progenitors did not correlate significantly with age, sex, or disease duration (P > .5 for all variables). Eleven patients treated with hydroxyurea had a trend toward reduced numbers of V617F-positive BFU-Es (P = .09), consistent with the observation that, compared with V617F-negative ET patients, blood counts of V617F-positive ET patients are particularly sensitive to hydroxyurea.6 The proportion of V617F-positive BFU-Es was significantly lower in patients with ET than in patients with PV (30.6% ± 27.1% vs 52.5% ± 26.6%, respectively; P = .02), an observation that did not reflect a shorter time from diagnosis (median disease durations of 59 months and 18 months, respectively). Moreover, whereas homozygous-mutant BFU-Es were found in all 17 patients with PV, none was detected in any of the 17 patients with ET (P < .001). In patients with PV, 129 of 335 V617F-positive BFU-Es were mutation homozygous compared with 0 of 248 in patients with ET (P < .001). Furthermore, 0 of 76 EECs in 3 patients with ET (patients 30, 33, and 34) were V617F homozygous, compared with 130 of 270 EECs from the patients with PV (P < .001). These data demonstrate that, in contrast to patients with PV, patients with ET rarely harbor V617F-homozygous erythroid progenitors, although the existence of rare patients with ET who have mutation-homozygous progenitors cannot be excluded.
Frequency of V617F homozygosity in patients with ET. (A) Frequencies of wild-type, V617F-heterozygous, and V617F-homozygous BFU-Es in the peripheral blood of 17 ET patients were determined using PCR and BsaXI digestion. The number of colonies genotyped from each patient is at the top of each column. (B) BsaXI digestion of JAK2 exon 14 and SCL intron 1 was used to genotype DNA from peripheral blood BFU-Es cultured from 2 ET patients following transformation to PV (patients 37 and 38). Lanes 1-10 show individual colonies; lane 11, undigested granulocyte DNA from a V617F-negative control; lane 12, BsaXI-digested granulocyte DNA from a V617F-negative control; and lane 13, water.
Frequency of V617F homozygosity in patients with ET. (A) Frequencies of wild-type, V617F-heterozygous, and V617F-homozygous BFU-Es in the peripheral blood of 17 ET patients were determined using PCR and BsaXI digestion. The number of colonies genotyped from each patient is at the top of each column. (B) BsaXI digestion of JAK2 exon 14 and SCL intron 1 was used to genotype DNA from peripheral blood BFU-Es cultured from 2 ET patients following transformation to PV (patients 37 and 38). Lanes 1-10 show individual colonies; lane 11, undigested granulocyte DNA from a V617F-negative control; lane 12, BsaXI-digested granulocyte DNA from a V617F-negative control; and lane 13, water.
There are at least 3 possible explanations for these striking results. First, these patients with PV are likely to have lower serum Epo levels than the patients with ET, and V617F-homozygous BFU-Es may have a selective advantage in a low-Epo environment. However, for individual patients with PV, the fractions of V617F-homozygous BFU-Es and CFU-GMs were similar, suggesting that selection for homozygosity occurred in multipotent progenitors and was therefore unlikely to be dependent on the Epo receptor, which is first expressed at the BFU-E stage of differentiation.14 Second, compared with patients with ET, patients with PV may have a longer prediagnosis phase of their disease, allowing more time for homozygosity to occur. Third, mitotic recombination involving the JAK2 locus may occur more frequently in patients with PV.
It is recognized that patients with ET may infrequently transform to PV.15,16 We therefore studied 2 patients who presented with V617F-positive ET and no evidence of erythrocytosis, but who developed PV 36 or 147 months after their initial diagnosis. V617F-homozygous BFU-Es and EECs were present in both patients after polycythemic transformation (P = .006; Figure 2B). Colony data from the time of their presentation with ET are not available, so we cannot exclude the possibility that these patients had V617F-homozygous colonies prior to transformation. However, taken together with the absence of detectable V617F-homozygous BFU-Es in all patients with ET, our results raise the possibility that, in some patients, duplication of the mutant JAK2 allele with loss of the normal allele may be associated with a phenotypic change from thrombocythemia to polycythemia.
Prepublished online as Blood First Edition Paper, June 13, 2006; DOI 10.1182/blood-2006-04-018259.
Supported by the UK Leukaemia Research Fund.
L.M.S. designed the experiments, processed and genotyped the hematopoietic colonies, performed sequencing and statistical analyses, and cowrote the manuscript; M.A.S. performed the hematopoietic colony assays; P.J.C. performed statistical analyses; and A.R.G. designed the experiments and cowrote the manuscript.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Drs Wendy Erber and Anthony Bench, together with the staff of the Addenbrooke's Haematological Disorder Sample Bank, for their support, and to Lynsey Joy for technical assistance.