Abstract
Transfusion-related acute lung injury (TRALI) is a form of posttransfusion acute pulmonary insufficiency that has been linked to the infusion of biologic response modifiers (BRMs), including antileukocyte antibodies and lipids. Soluble CD40 ligand (sCD40L) is a platelet-derived proinflammatory mediator that accumulates during platelet storage. We hypothesized that human polymorpho-nuclear leukocytes (PMNs) express CD40, CD40 ligation rapidly primes PMNs, and sCD40L induces PMN-mediated cytotoxicity of human pulmonary microvascular endothelial cells (HMVECs). Levels of sCD40L were measured in blood components and in platelet concentrates (PCs) implicated in TRALI or control PCs that did not elicit a transfusion reaction. All blood components contained higher levels of sCD40L than fresh plasma, with apheresis PCs evidencing the highest concentration of sCD40L followed by PCs from whole blood, whole blood, and packed red blood cells (PRBCs). PCs implicated in TRALI reactions contained significantly higher sCD40L levels than control PCs. PMNs express functional CD40 on the plasma membrane, and recombinant sCD40L (10 ng/mL-1 μg/mL) rapidly (5 minutes) primed the PMN oxidase. Soluble CD40L promoted PMN-mediated cytotoxicity of HMVECs as the second event in a 2-event in vitro model of TRALI. We concluded that sCD40L, which accumulates during blood component storage, has the capacity to activate adherent PMNs, causing endothelial damage and possibly TRALI in predisposed patients.
Introduction
CD40 is a 48-kDa transmembrane glycoprotein and a member of the tumor necrosis factor (TNF) receptor family expressed on endothelial and epithelial cells, monocytes, and macrophages.1 CD40 ligand (CD40L [CD154]) is a primarily platelet-derived pro-inflammatory mediator found in soluble (sCD40L) and cell-associated forms in transfused blood.2,3 Soluble CD40L activates macrophages and elicits the production and release of multiple proinflammatory cytokines.4 Furthermore, inhibition of the CD40-CD40L system in animal models reduces acute lung injury (ALI) caused by endotoxin (lipopolysaccharide [LPS]) or oxygen toxicity.5-7 In addition, sCD40L is present in platelet concentrates and accumulates over routine 3- to 5-day storage times.3
Polymorphonuclear leukocytes (PMNs) are critical in host defense against pathogens and exert their major microbicidal function in the tissues.8,9 PMN priming is initiated by the attraction and adhesion of PMNs to activated vascular endothelium and continues until the pathogens are phagocytosed and destroyed.6,10-12 PMN-mediated acute lung injury (ALI) requires at least 2 separate events: endothelial activation, which includes the synthesis and release of chemokines and the increased surface expression of adhesion molecules that elicit PMN adhesion, and activation of adherent PMNs, which causes the release of their microbicidal arsenal and results in endothelial damage, capillary leak, and ALI.10,11,13-16 Such a 2-event model has been proposed for ALI, especially for transfusion-related acute lung injury (TRALI).14,16,17 The 2-event model of TRALI postulates that the first event is the clinical status of the patient leading to pulmonary sequestration of PMNs and that the second is the infusion of biologic response mediators (BRMs), including antibodies directed against PMN antigens or lipids, leading to the activation of PMNs and the release of cytotoxic substances and PMN-mediated ALI.11,14-16 We hypothesized that PMNs express CD40 and ligation causes changes in PMN function, that transfusion of cellular products containing sCD40L rapidly primes PMNs, and that sCD40L can serve as a second event in the 2-event model of TRALI, in which sCD40L causes PMN-mediated cytotoxicity of pulmonary endothelium.
Patients, materials, and methods
Materials
All reagents, unless otherwise specified, were purchased from Sigma Chemical (St Louis, MO). Solutions were made from sterile water for injection (United States Pharmacopeia; Baxter Healthcare, Deerfield, IL). All buffers were made from the following stock USP solutions: 10% CaCl2, 23.4% NaCl, 50% MgSO4 (American Reagent Laboratories, Shirley, NY); sodium phosphates (278 mg/mL monobasic and 142 mg/mL dibasic), and 50% dextrose (Abbott Laboratories, North Chicago, IL). Furthermore, all solutions were sterile-filtered with Nalgene MF75 series disposable sterilization filter units purchased from Fisher Scientific (Pittsburgh, PA). Ficoll-Paque was purchased from Amersham Biosciences (Piscataway, NJ). Plastic microplates, manufactured by Nunc, T-25 tissue-culture flasks, 12-well plates, and sterile pipettes were purchased from Life Sciences Products (Frederick, CO). Human pulmonary microvascular endothelial cells (HMVECs) and all media and tissue-culture reagents were obtained from Cambrex (Walkersville, MD). Recombinant sCD40L and sCD40L (human) ELISA kits were obtained from R&D Systems (Minneapolis, MN). Murine antibodies to CD40 and goat anti–mouse F(ab′)2 fragments were purchased from BioSource International (Camarillo, CA).
Patient population
All patients included within the study satisfied the definition of TRALI as previously published.16 In addition, all TRALI reactions met the clinical criteria for this diagnosis delineated in a National Institutes of Health (NIH) consensus panel in June 2003 and the Canadian Consensus Conference in April 2004.18,19 Informed consent was obtained from all study patients through an institutional review board–approved protocol from the University of Alberta (Edmonton, Canada) prior to enrollment in this study.
Measurement of sCD40L
Soluble CD40L concentrations in human plasma or the plasma fraction of whole blood (WB) or blood components were measured in duplicate with a modified ELISA protocol that captured monomeric, dimeric, and multimeric forms of sCD40L. Briefly, a 96-well plate was coated with 3 μg/mL mouse anti–human sCD40L antibody (a gift from Marilyn Kerry, University of Rochester, Rochester, NY) and were allowed to incubate overnight at room temperature. A blocking buffer consisting of PBS, 1% BSA (fraction V), and 0.1% NaN3 was added to the ELISA plate and allowed to incubate for 1 hour. The plate was then washed 3 times with a washing buffer consisting of PBS, 0.05% Tween 20, and 0.1% NaN3. Recombinant human CD40L (Bender Med Systems, Vienna, Austria) was added to each plate with 1 to 2 serial dilutions in blocking buffer to create a standard curve of 6.25 to 400 pg/mL. ELISA plates were allowed to incubate for 2 hours and were washed 3 times with washing buffer. Mouse anti–human CD40L biotinylated antibody (Ancell, Bayport, MN) was added to each well (2 μg/mL in blocking buffer) and allowed to incubate 2 hours for capture. ELISA plates were then washed 3 times with washing buffer. Streptavidin–alkaline phosphatase (1:100; Bio-Rad Laboratories, Hercules, CA) was added to each well and allowed to incubate for 30 minutes before it was washed 3 times. The ELISA plates were then developed using an alkaline phosphatase substrate kit (Bio-Rad Laboratories) according to the manufacturer's directions. After 30 minutes of incubation, optical density was determined at a wavelength of 405 nm with a reference of 610 nm.
Soluble CD40L concentrations were measured in fresh plasma (FP), fresh frozen plasma (FFP), whole blood (WB) plasma, packed red blood cells (PRBCs) leukoreduced or unmodified before storage, and platelet concentrates (PCs) collected by apheresis techniques (A-Plts). For studies of PRBCs, 5 healthy subjects donated 450 mL WB that was separated into PRBCs by standard techniques according to American Association of Blood Banks (AABB) criteria.20 Half the PRBC units (50% by weight) were leukoreduced (LR) before storage with the use of a Hemasure r/Ls filter (Whatman, Maidstone, United Kingdom), the standard leukoreduction filter used at Bonfils Blood Center (Denver, CO), and the other half was unmodified (nonleukoreduced [NLR]; PRBCs) before storage, and samples were taken through a sterile coupler at days 1, 28, and 42 (the last day the unit may be transfused). Healthy human volunteers donated 1 U each of A-Plts (Cobe Trima; Gambro BCT, Lakewood, CO) after informed consent had been obtained in accordance with the Human Subjects Committee of the University of Colorado School of Medicine. A-Plts were stored according to AABB standards, and samples (50-75 mL) were obtained through sterile couplers (Baxter, Deerfield, IL) on days 1, 3, 5 (the last day the unit could be transfused), and 7.16,20 All platelet units received standard bacterial cultures before release for sampling, and all units or bags were visually inspected for bacterial growth during the storage interval and before they were discarded.20 Leukocyte counts were also performed on all 5 units, and each unit had fewer than 0.5 × 106 leukocytes. FP samples from 5 healthy donors (3 women, 2 men) were obtained through standard venipuncture using 18-gauge butterfly needles connected to syringes. Citrated whole blood was then centrifuged at 5000g for 7 minutes to remove cellular debris; this was followed by a second centrifugation step at 12 500g for 5 minutes to remove acellular debris.21 Plasma was removed, aliquoted, and stored at –70°C. In addition, plasma samples from FFP, NLR-PRBCs, LR-PRBCs, WB-Plts, and A-Plts were obtained through sterile couplers and underwent separation identical to that for FP, and concentrations of sCD40L were measured in these samples using FP and FFP as controls on each ELISA plate.
Soluble CD40L concentrations were also quantified in PCs implicated in TRALI reactions, derived from WB-Plts or A-Plts, compared with control WB-Plt concentrates stored for an identical length of time that did not cause transfusion reactions when infused into hospital patients16 or compared with 5 U A-Plts stored for an identical length of time. Samples from TRALI patients and the implicated platelet units, either WB-Plts or A-Plts, were drawn immediately when the reaction was recognized and the transfusions were stopped. Patient samples and remaining implicated platelet units were transported to the Blood Bank (University of Alberta Hospital) and were received within 60 minutes of the TRALI reaction (these reactions were part of an IRB-approved study under the direction of Dr Lynn Boshkov, University of Alberta Hospital). Transportation of all samples occurred within the hospital without notable temperature variation, and the plasma was separated, aliquoted, and frozen at –70°C within 2 hours of identification of a possible TRALI reaction. Informed consent was obtained for all control and TRALI patients included in this study. Samples from control patients who received platelet transfusions and who did not have transfusion reactions were treated in an identical manner. Before measurement of the sCD40L concentration, the samples were thawed and centrifuged at 12 500g for 5 minutes.
PMN isolation
Heparinized whole blood was drawn from healthy human donors, after informed consent, with the use of a protocol approved by the Colorado Multiple Institutional Review Board. PMNs were isolated by standard techniques, including dextran sedimentation, Ficoll-Hypaque gradient centrifugation, and hypotonic lysis of contaminating red blood cells.21 Whole cell lysates were made by sonication and centrifugation (700g) to obtain the postnuclear supernatant and then were boiled in Laemmli–SDS lysis buffer, as previously reported.22,23 We also obtained whole cell lysates as previously described.22
CD40 expression in PMNs and activation by antibody cross-linking
The presence of CD40 on PMNs was initially determined by Western blot analysis of whole cell lysates. Cross-linking of CD40 on PMNs was accomplished by incubation of PMNs with a primary mouse monoclonal antibody to CD40 followed by incubation with goat F(ab′)2 antimouse fragments for 5 minutes at 37°C, pH 7.35.24 PMNs were then activated by 1 μM formyl-Met-Leu-Phe (fMLP), and the maximal rate of superoxide anion release was measured as the reduction of cytochrome c, as previously published.21 Priming activity was determined as the augmentation of the fMLP-activated respiratory burst in control cells (eg, PMNs treated with isotypic antibody controls).7,21
Digital fluorescence microscopy
PMNs (5 × 105) were warmed to 37°C and prepared as previously described.12 Images were acquired with a Zeiss Axiovert microscope (Carl Zeiss, Oberkochen, Germany) fitted with a 63 ×/1.4 NA oil-immersion objective lens (Carl Zeiss) and a Cooke CCD SensiCam (Applied Scientific Instrumentation, Eugene, OR) using a Chroma Sedat Multiple Bandpass filter wheel (Chroma Technology, Brattleboro, VT) and a Sutter filter control (BD Biosciences Bioimaging, Rockville, MD). Images were acquired with Slidebook 4.1 software (Intelligent Imaging Innovations, Denver, CO). All images compared within a single figure were acquired as Z-stacks in 0.2-μm intervals. All Z-stack images were deconvolved by the application of constrained iterative deconvolution and Gaussian noise smoothing from system-specific point spread functions.25 After deconvolution, images were cropped to represent the middle-most planes (center ± 10 planes), and the proteins in question were masked to represent zero fluorescence in IgG-negative controls, prepared as previously described.12 In short, slides were incubated with a mouse IgG isotype, with the host species as the primary antibody against CD40. Positive binding of the CD40 antibody was determined by setting the lowest fluorescence threshold to a value identical to the greatest fluorescence threshold seen from the negative controls. Fluorescence of CD40 (red) was detected with the use of a donkey antimouse secondary antibody conjugated with Cy3, which binds directly to the monoclonal antibody to CD40. The membrane was identified by the association of the lectin wheat germ agglutinin (WGA) to salicylic acid residues linked to the outer surfaces of membrane proteins.26 WGA was conjugated with AlexaFluor 488 (Ex-495 Em-519; Invitrogen, Carlsbad, CA) and was acquired on the FITC channel (green). The nucleus was identified by the DNA-specific stain bis-benzimide, which was fluorescent (blue) and acquired on the Cy5 channel.26,27 Overlay images were displayed in pseudocolor with the Slidebook (Intelligent Imaging Innovations).
PMN priming
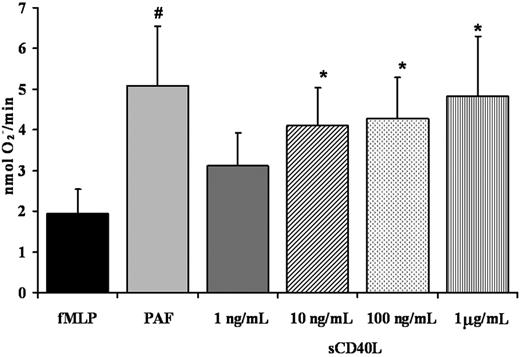
PMN priming assays were completed as follows: PMNs (3.75 × 105) were incubated with buffer (Krebs-Ringers phosphate with 2% dextrose [KRPD]) controls, sCD40L (1 ng/mL, 10 ng/mL, 100 ng/mL, and 1 μg/mL), or 2 μM platelet-activating factor (PAF) for 3, 5, or 15 minutes at 37°C, followed by activation with 1 μM fMLP, and the maximal rate of superoxide production was measured.28 Priming activity was calculated as the augmentation of the maximal rate of O2– in response to fMLP.
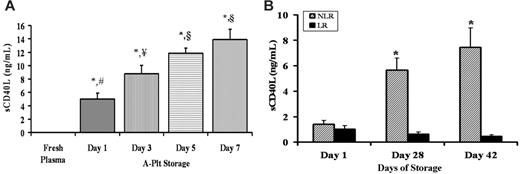
Accumulation of sCD40L in stored A-Plts and LR-PRBCs and NLR-PRBCs. (A) A-Plts were serially stored according to AABB standards, plasma samples were obtained and separated from the cells by centrifugation, sCD40L concentration (ng/mL) was measured by commercial ELISA, and fresh, heparinized plasma taken from healthy donors was used as a control for sCD40L concentration. The sCD40L concentration in A-Plts was significantly greater at days 1, 3, 5, and 7 of storage in the A-Plts than in FP (P < .05; n = 5). Moreover, the levels continued to significantly increase on days 3, 5, and 7 compared with day 1. However, the concentrations of sCD40L on day 5 compared with day 7 were not significantly different from one another (P < .05). *Statistical significance from FP (P < .05); ¥,§Significance from day 1 (#) (P < .05). (B) Accumulation of sCD40L in PRBCs in which 50% of the unit (by weight) was leukoreduced (LR) and 50% was unmodified (NLR) before storage. sCD40L concentrations (ng/mL) were measured serially in LR-PRBCs and NLR-PRBCs on days 1, 28, and 42. Day 28 and day 42 NLR (hatched bars) were statistically significant (P < .05; n = 5) compared with day 1 NLR. Days 28 and 42 NLR were also significant from days 1, 28, and 42 LR (solid bars). *Statistical significance (P < .05) compared with day 1 of storage.
Accumulation of sCD40L in stored A-Plts and LR-PRBCs and NLR-PRBCs. (A) A-Plts were serially stored according to AABB standards, plasma samples were obtained and separated from the cells by centrifugation, sCD40L concentration (ng/mL) was measured by commercial ELISA, and fresh, heparinized plasma taken from healthy donors was used as a control for sCD40L concentration. The sCD40L concentration in A-Plts was significantly greater at days 1, 3, 5, and 7 of storage in the A-Plts than in FP (P < .05; n = 5). Moreover, the levels continued to significantly increase on days 3, 5, and 7 compared with day 1. However, the concentrations of sCD40L on day 5 compared with day 7 were not significantly different from one another (P < .05). *Statistical significance from FP (P < .05); ¥,§Significance from day 1 (#) (P < .05). (B) Accumulation of sCD40L in PRBCs in which 50% of the unit (by weight) was leukoreduced (LR) and 50% was unmodified (NLR) before storage. sCD40L concentrations (ng/mL) were measured serially in LR-PRBCs and NLR-PRBCs on days 1, 28, and 42. Day 28 and day 42 NLR (hatched bars) were statistically significant (P < .05; n = 5) compared with day 1 NLR. Days 28 and 42 NLR were also significant from days 1, 28, and 42 LR (solid bars). *Statistical significance (P < .05) compared with day 1 of storage.
Two-event in vitro model of PMN-mediated pulmonary endothelial damage
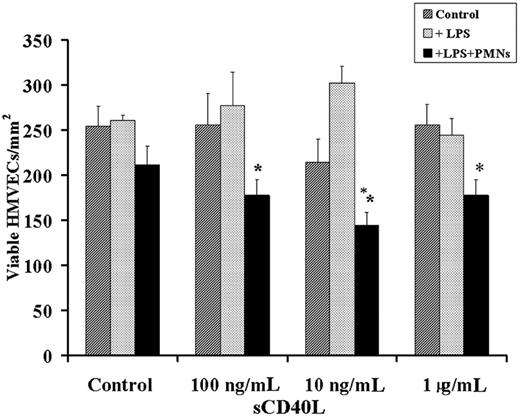
A 2-event in vitro model of PMN-mediated pulmonary endothelial damage was performed as previously described.17 Briefly, HMVECs were grown to 80% to 90% confluence on 12-well plates and incubated with 2 μg/mL endotoxin from Salmonella enteritides LPS or buffer for 6 hours at 37°C in 5% CO2. PMNs (1 × 106) were added at a 10:1 effector-cell–to–target-cell ratio and were allowed to settle for 30 minutes. After settling, the PMNs were exposed to buffer or sCD40L (10 ng/mL-1 μg/mL) for 30 minutes. Supernatants were removed by quickly inverting and forcefully decanting the plates from a height of 50 cm onto absorbent towels.17 After the addition of warm KRPD, the number of viable HMVECs, adherent and trypan blue negative, were counted over a 4-mm2 surface area by 3 separate observers to exclude bias. Controls consisted of HMVECs alone without PMNs or incubated with all reagents used in these experiments, alone or in combination, and viability was assessed.
Statistical analysis
Statistical analysis was completed with the use of repeated-measures analysis of variance (ANOVA) followed by post hoc Bonferroni test for multiple comparisons. Significance was determined at a P value below .05.
Results
Soluble CD40L accumulation in blood components
Because sCD40L accumulation has only been evaluated at the outdate (the last day the unit may be transfused) of platelet concentrates from A-Plts (day 5), we measured sCD40L concentration serially in the plasma from A-Plts with storage time to day 7 and in other blood components including FP and FFP and the plasma fractions from WB, WB-Plts, and PRBCs (both LR and NLR).3,26,29 Compared with FP from healthy volunteers (3 women, 2 men), the amount of sCD40L from the plasma fraction of A-Plts was increased on all days of storage (days 1-7); moreover, sCD40L concentrations in A-Plts increased significantly on days 3, 5, and 7 of storage compared with day 1 (day 1, 5.0 ± 0.8 ng/mL; day 3, 8.8 ± 1.2 ng/mL; day 5, 11.8 ± 0.8 ng/mL; day 7, 14.0 ± 1.5 ng/mL; P < .05) (Figure 1A). However, the concentration of sCD40L on day 7 was not significantly greater than the sCD40L accumulation measured on day 5 (Figure 1A). Soluble CD40L was also measured in the plasma fraction of LR- and NLR-stored PRBCs on days 1, 28, and 42 (Figure 1B). Compared with the FP from healthy donors, a mild but significant elevation in sCD40L was observed in the plasma fraction of LR-PRBCs and NLR-PRBCs; moreover, the levels of sCD40L increased in the plasma fraction of NLR-PRBCs throughout storage and were significantly higher than concentrations from LR-PRBCs at days 28 and 42 (*P < .05) (day 28: LR 0.62 ± 0.17 ng/mL vs NLR 5.6 ± 0.9*; day 42: LR 0.45 ± 0.1 vs NLR 7.4 ± 1.5*) (Figure 1B). These data demonstrate that sCD40L levels were elevated in NLR-PRBCs compared with LR-PRBCs. Because LR-PRBCs had much lower levels of sCD40L than NLR-PRBCs, we investigated whether the Hemasure r/LS leukoreduction filters removed platelets as well as leukocytes. In a study of 11 whole blood donations, 50% of the PRBCs were filtered and the other 50% were left as an unmodified control (NLR-PRBCs). The Hemasure rL/S filters significantly removed platelets from LR-PRBCs by almost 2 logs compared with NLR-PRBCs (3.36 ± 0.69 Plt × 103/μL [LR-PRBCs] vs 285.55 ± 27.31 Plt × 103/μL [NLR-PRBCs]; P < .01). Lastly, sCD40L concentrations were also measured in 5 U WB at component outdate and in 5 U FFP, and these concentrations were significantly elevated compared with FP levels from 5 healthy donors (15.0 ± 2.0 ng/mL [WB] and 1.3 ± 0.1 ng/mL [FFP] vs 0.01 ± 0.003 ng/mL [FP]; *P < .05).
Plasma sCD40L concentrations in implicated platelet concentrates and in TRALI patients before and after transfusion
Soluble CD40L concentrations were measured in WB-Plts (n = 62) implicated in TRALI reactions and were compared with WB-Plts infused into hospital patients (n = 59). Platelet concentrates were stored for identical amounts of time and did not cause transfusion reactions (Table 1). In addition, sCD40L concentrations were measured in the plasma of A-Plts implicated in TRALI (n = 29) and were compared with 5 control A-Plts units, stored for identical amounts of time, and were not infused (Table 1). In both cases, the platelet concentrates implicated in TRALI reactions, whether WB derived or collected by apheresis, had significantly greater concentrations of sCD40L in the plasma fraction than did control platelet concentrates stored for an identical period of time (P < .05) (Table 1). These data represent the results from individual A-Plt and WB-Plt units and from pooled WB-Plts, depending on the products transfused; however, all units infused into TRALI patients or control subjects were tested without exception. Moreover, if one takes into account the total amount of sCD40L infused into the TRALI patients from the implicated WB-Plt concentrate units compared with the control WB-Plt concentrate units, the TRALI patients were exposed to 4.4 ± 0.4 μg sCD40L compared with 2.5 ± 0.3 μg in control patients, which demonstrates that TRALI patients received significantly larger amounts of sCD40L than control subjects (P < .05).
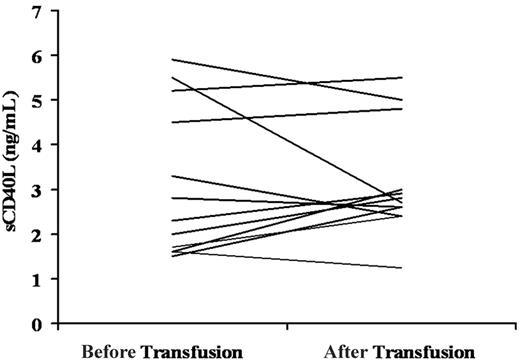
Soluble CD40L concentration in the plasma of TRALI patients before and after transfusion. Each line reflects the sCD40L in a pretransfusion typing plasma (left) compared with the sCD40L concentration in a plasma sample drawn when TRALI was clinically recognized (right).16 In 8 of 12 TRALI patients, levels of sCD40L concentrations increased at the time TRALI was diagnosed.
Soluble CD40L concentration in the plasma of TRALI patients before and after transfusion. Each line reflects the sCD40L in a pretransfusion typing plasma (left) compared with the sCD40L concentration in a plasma sample drawn when TRALI was clinically recognized (right).16 In 8 of 12 TRALI patients, levels of sCD40L concentrations increased at the time TRALI was diagnosed.
Soluble CD40L concentrations were also measured in 12 TRALI patients' plasma samples before and after transfusion, and the circulating levels of sCD40L increased in 8 of 12 patients at the time TRALI was clinically recognized after transfusion (3.3 ng/mL) compared with the levels in the pretransfusion plasma level (3.1 ng/mL) (Figure 2). The increased level of sCD40L was not statistically significant from sCD40L concentrations in the pretransfusion plasma samples.
PMNs express CD40 on the plasma membrane
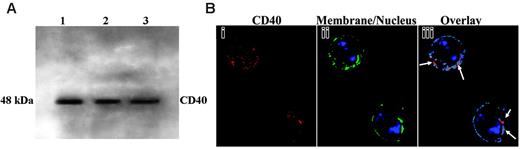
Because the presence of CD40 has not been investigated in PMNs, CD40 expression was initially determined by immunoblotting proteins from whole cell lysates, which demonstrated a band of immunoreactivity at 48 kDa, the reported molecular weight of CD401 (Figure 3A). Fixed PMNs were then smeared onto slides and incubated with human CD40 antibody, and images were prepared as previously reported.12,29 Compared with the negative controls consisting of fluorescently labeled isotypic immunoglobulins, CD40 immunoreactivity was present at the periphery of the cell (Figure 3B). Colocalization of CD40 at the plasma membrane is displayed in pseudocolor; red represents high amounts of colocalization, and blue represents low colocalization between CD40 and the plasma membrane (Figure 3B).
PMNs express an active CD40 receptor
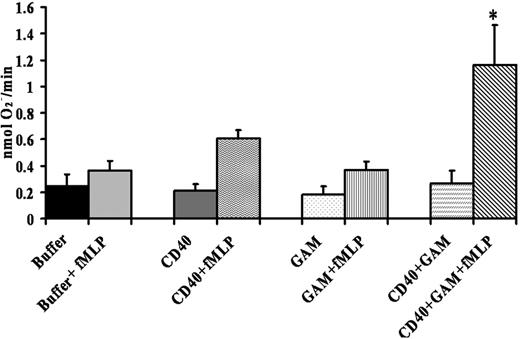
To determine whether the CD40 receptor was functional, isolated PMNs were incubated with a monoclonal antibody to CD40, isotypic antibody controls, or buffer, followed by the addition of goat anti–mouse F(ab′)2 fragments to cross-link the anti–CD40 antibody bound to CD40. Admixtures were then activated by 1 μM fMLP, and the maximal rate of superoxide anion was measured. These data demonstrated that cross-linking of antibodies specific for CD40 caused significant priming (P < .05) of the fMLP-activated PMN oxidase compared with the isotypic controls alone, CD40 antibodies alone, or PMNs incubated with buffer plus goat anti–mouse F(ab′)2 or PMNs incubated with isotype controls plus goat anti–mouse F(ab′) (Figure 4).
Expression of CD40 in human PMNs by Western blot analysis and localization of CD40 by digital microscopy. (A) Experiments were performed on 3 donors. Proteins from PMN whole cell lysates (1.25 × 106) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and were immunoblotted with a monoclonal antibody to human CD40. The CD40 glycoprotein band is present at 48 kDa. (B) Fixed PMNs were incubated with a human CD40 monoclonal antibody, and then the images were prepared for digital microscopy as previously described.12 (i) CD40 immunoreactivity is red because of the secondary donkey antimouse antibody conjugated to Cy3. (ii) The membrane is green because of the association of the wheat germ agglutinin (WGA)–AlexaFluor 488 with salicylic acids, and the nucleus is stained blue with the fluorescent bis-benzimide nuclear dye. (iii) Colocalization of CD40 and the plasma membrane is displayed in pseudocolor. Arrows point to the red area, where a high amount of colocalization is present. Blue represents low colocalization between CD40 and the plasma membrane. These images are representative of 3 different experiments on the PMNs of 3 donors.
Expression of CD40 in human PMNs by Western blot analysis and localization of CD40 by digital microscopy. (A) Experiments were performed on 3 donors. Proteins from PMN whole cell lysates (1.25 × 106) were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and were immunoblotted with a monoclonal antibody to human CD40. The CD40 glycoprotein band is present at 48 kDa. (B) Fixed PMNs were incubated with a human CD40 monoclonal antibody, and then the images were prepared for digital microscopy as previously described.12 (i) CD40 immunoreactivity is red because of the secondary donkey antimouse antibody conjugated to Cy3. (ii) The membrane is green because of the association of the wheat germ agglutinin (WGA)–AlexaFluor 488 with salicylic acids, and the nucleus is stained blue with the fluorescent bis-benzimide nuclear dye. (iii) Colocalization of CD40 and the plasma membrane is displayed in pseudocolor. Arrows point to the red area, where a high amount of colocalization is present. Blue represents low colocalization between CD40 and the plasma membrane. These images are representative of 3 different experiments on the PMNs of 3 donors.
Recombinant soluble CD40L primes the PMN oxidase
Given that PMNs express functional CD40, we investigated the ability of recombinant sCD40L to prime the fMLP-activated oxidase in human PMNs. The sCD40L priming activity was measured over a range of incubation times (3-15 minutes) and concentrations (1 ng/mL-5 μg/mL). Significant priming activity of sCD40L compared with buffer-treated controls was present at 3 and 5 minutes, with priming of the fMLP-activated respiratory burst reaching a relative maximum at 5 minutes (P < .05) (Figure 5). At longer incubation times (10-15 minutes), the priming activity decayed and was not significantly increased at 15 minutes compared with identical buffer-treated controls (results not shown). With respect to concentration, sCD40L significantly primed PMNs at a range of concentrations from 10 ng/mL to 1 μg/mL compared with buffer-treated controls (P < .05) (Figure 5). In addition, preincubation of PMNs with antibodies specific for CD40 inhibited the priming activity of sCD40L by 50% ± 7%.
Cross-linking CD40 primes the PMN oxidase. PMNs were incubated with a mouse monoclonal antibody to human CD40, isotype controls, or buffer for 60 minutes at 4°C, followed by the addition of a buffer control or a goat anti–mouse F(ab′)2 (GAM) to cross-link the CD40 antibody. Activation of the PMN oxidase was accomplished with the addition of 1 μM fMLP, and priming of the fMLP-activated oxidase was calculated as the augmentation of the maximal rate of O2– in response to fMLP in buffer-treated controls. *Significant increase in oxidase activity compared with the buffer-treated controls (P < .05). The only group to significantly prime the fMLP-activated respiratory burst was CD40 + GAM + fMLP, indicating that cross-linking CD40 directly causes priming of the NADPH oxidase. Importantly, incubation of PMNs treated with isotype controls with the F(ab′)2 cross-linkers did not cause priming of the fMLP-activated oxidase (results not shown). This figure is representative of 7 separate experiments using disparate blood donors as the source of the PMNs. Data are presented as mean ± standard error of the mean.
Cross-linking CD40 primes the PMN oxidase. PMNs were incubated with a mouse monoclonal antibody to human CD40, isotype controls, or buffer for 60 minutes at 4°C, followed by the addition of a buffer control or a goat anti–mouse F(ab′)2 (GAM) to cross-link the CD40 antibody. Activation of the PMN oxidase was accomplished with the addition of 1 μM fMLP, and priming of the fMLP-activated oxidase was calculated as the augmentation of the maximal rate of O2– in response to fMLP in buffer-treated controls. *Significant increase in oxidase activity compared with the buffer-treated controls (P < .05). The only group to significantly prime the fMLP-activated respiratory burst was CD40 + GAM + fMLP, indicating that cross-linking CD40 directly causes priming of the NADPH oxidase. Importantly, incubation of PMNs treated with isotype controls with the F(ab′)2 cross-linkers did not cause priming of the fMLP-activated oxidase (results not shown). This figure is representative of 7 separate experiments using disparate blood donors as the source of the PMNs. Data are presented as mean ± standard error of the mean.
PMN-mediated HMVEC damage
Because PMN-mediated injury of pulmonary endothelium is thought to require at least 2 separate cellular events, we developed an in vitro model of PMN-mediated pulmonary HMVEC damage that required HMVEC activation leading to PMN adherence and activation of these adherent PMNs resulting in the activation and release of cytotoxic agents from the microbicidal arsenal and the killing of HMVECs.17 HMVECs were incubated for 6 hours with buffer or 2 μg/mL LPS, which resulted in proinflammatory activation of these endothelial cells as documented by the synthesis and release of chemokines, specifically IL-8, GRO-α, and ENA-78, and increased ICAM-1 surface expression compared with the buffer-treated PMNs.17 LPS-induced adherence was significantly greater (P < .05; n = 6) than in buffer-treated controls (18.4% ± 3.5% PMNs [LPS] vs 1.5% ± 0.6% PMNs [buffer controls]). This LPS-induced adhesion could be abrogated by preincubation of the HMVECs with antibodies to adhesion molecules or immunoglobulin neutralization of the released chemokines (18.4% ± 3.5% [LPS] vs 1.5% ± 0.9% [anti-CD18] and vs 1.8% ± 0.5% PMNs [chemokine neutralization]) and was not different from PMN adherence to buffer-treated HMVECs (1.5% ± 0.6%), identical to previous results.17,24 The addition of sCD40L (10 ng/mL-1 μg/mL) to LPS-activated HMVECs with or without PMNs resulted in significant HMVEC cytotoxicity compared with buffer-treated HMVEC controls with or without PMNs activated with sCD40L or buffer, LPS-activated HMVECs with or without PMNs activated with buffer, and buffer or LPS-activated HMVECs with sCD40L (Figure 6). Furthermore, the concentrations of sCD40L that caused significant neutrophil oxidase priming caused extensive HMVEC killing. Lastly, HMVEC viability was not affected by any reagents, alone or in combination, without PMNs (Figure 6 and results not shown; data available on request).
Soluble CD40L primes the PMN oxidase. PMN priming assays were completed with incubation of PMNs with buffer, sCD40L (1 ng/mL-1 μg/mL), or PAF (2 μM) (positive control). The ability of sCD40L to prime the fMLP-activated oxidase in human PMNs was measured over a range of concentrations for 5 minutes. Significant priming of the PMN oxidase occurred with the addition of 10 ng/mL, 100 ng/mL, and 1 μg/mL sCD40L compared with buffer-treated controls (#P < .05; *P < .05; n = 7); significant differences exist between groups with different symbols. Data are presented as mean ± standard error of the mean.
Soluble CD40L primes the PMN oxidase. PMN priming assays were completed with incubation of PMNs with buffer, sCD40L (1 ng/mL-1 μg/mL), or PAF (2 μM) (positive control). The ability of sCD40L to prime the fMLP-activated oxidase in human PMNs was measured over a range of concentrations for 5 minutes. Significant priming of the PMN oxidase occurred with the addition of 10 ng/mL, 100 ng/mL, and 1 μg/mL sCD40L compared with buffer-treated controls (#P < .05; *P < .05; n = 7); significant differences exist between groups with different symbols. Data are presented as mean ± standard error of the mean.
A 2-event in vitro model of PMN-mediated pulmonary endothelial damage. HMVECs were grown to 80% to 90% confluence on 12-well plates and were incubated with buffer or LPS for 6 hours, followed by the addition of buffer or freshly isolated PMNs that were allowed to settle for 30 minutes at a target-effector ratio of 10:1. After the addition of PMNs or buffer, a range of human recombinant CD40L concentrations or buffer controls was added to the HMVECs. (dark gray bars) Control cells; buffer-treated HMVECs with no PMNs. (light gray bars) HMVECs pretreated with 2 μg/mL LPS for 6 hours at 37°C, followed by treatment with buffer or a range of sCD40L concentrations (as delineated on the x-axis). (black bars) HMVECs incubated with LPS for 6 hours, followed by the addition of PMNs and sCD40L. Incubation of the adherent PMNs with concentrations of sCD40L at 100 ng/mL, 10 ng/mL, and 1 μg/mL resulted in a significant decrease in the number of viable HMVECs. *P < .05 (n = 3) compared with buffer. These data demonstrate that sCD40L may cause concentration-dependent PMN-mediated damage of LPS-activated HMVECs. Importantly, HMVECs stimulated with LPS and exposed to PMNs without sCD40L did not demonstrate PMN-mediated damage but did evidence widespread PMN adherence. Data are presented as mean ± standard error of the mean.
A 2-event in vitro model of PMN-mediated pulmonary endothelial damage. HMVECs were grown to 80% to 90% confluence on 12-well plates and were incubated with buffer or LPS for 6 hours, followed by the addition of buffer or freshly isolated PMNs that were allowed to settle for 30 minutes at a target-effector ratio of 10:1. After the addition of PMNs or buffer, a range of human recombinant CD40L concentrations or buffer controls was added to the HMVECs. (dark gray bars) Control cells; buffer-treated HMVECs with no PMNs. (light gray bars) HMVECs pretreated with 2 μg/mL LPS for 6 hours at 37°C, followed by treatment with buffer or a range of sCD40L concentrations (as delineated on the x-axis). (black bars) HMVECs incubated with LPS for 6 hours, followed by the addition of PMNs and sCD40L. Incubation of the adherent PMNs with concentrations of sCD40L at 100 ng/mL, 10 ng/mL, and 1 μg/mL resulted in a significant decrease in the number of viable HMVECs. *P < .05 (n = 3) compared with buffer. These data demonstrate that sCD40L may cause concentration-dependent PMN-mediated damage of LPS-activated HMVECs. Importantly, HMVECs stimulated with LPS and exposed to PMNs without sCD40L did not demonstrate PMN-mediated damage but did evidence widespread PMN adherence. Data are presented as mean ± standard error of the mean.
Discussion
The data presented demonstrate that sCD40L accumulated in the plasma fraction of most cellular blood components (WB, PCs, and PRBCs) and that sCD40L concentrations accumulated in A-Plts to higher concentrations than any other blood product. In addition, prestorage leukoreduction significantly decreased sCD40L levels, which may be explained by an almost 2-log reduction of contaminating platelets by the Hemasure r/LS filters used in these experiments. Moreover, sCD40L levels were increased in PCs implicated in TRALI reactions compared with the levels in control PCs that did not cause transfusion reactions and were stored for an identical length of time. Furthermore, increased posttransfusion levels of sCD40L were found in the plasma from 8 of 12 patients with documented TRALI. Because PMNs are the effector cells in TRALI15,16 and CD40 expression had not been demonstrated in PMNs, we determined that human PMNs express an active CD40 receptor and that receptor ligation, especially with sCD40L, can activate this receptor, which caused priming of the fMLP-activated oxidative burst. In addition, recombinant sCD40L could also serve as the second event in a well-characterized 2-event in vitro model of PMN-mediated endothelial damage.17 Importantly, our previous data demonstrated that recombinant, monomeric sCD40L, identical to that used in the priming and PMN-mediated HMVEC damage experiments, has decreased biologic activity compared with native (trimeric or dimeric) CD40L such that the supernatant of stored platelets stimulate lung fibroblast release of IL-6 and IL-8 partially inhibited by anti-CD154, an activity displayed to a lesser degree by recombinant sCD40L.3,30,31 The data presented demonstrate that, at physiologic levels, native sCD40L or even monomeric sCD40L could cause priming of the PMN oxidase and could serve as the second event in an in vitro model of PMN-mediated pulmonary endothelial damage. This in vitro model of PMN-mediated pulmonary endothelial damage required a first event to activate the pulmonary endothelium, resulting in PMN adherence (priming) similar to sequestration in the lung in vivo.15,17 In this model, LPS was used as the first event to mimic sepsis, a clinical condition that predisposes patients to TRALI and to other forms of ALI.14,17,31 Soluble CD40L, over a range of concentrations, could serve as the second event and could cause activation of the microbicidal arsenal of the adherent primed PMNs, resulting in HMVEC damage. Thus, the infusion of platelet concentrates or other blood components with significant amounts of sCD40L may have the capacity to cause TRALI in a susceptible host.
Soluble CD40L concentrations in all cellular blood components were significantly greater on day 1 than FP from healthy volunteers. This increase appeared to be caused by the isolation of the component itself from the activation of contaminating platelets, either by the tubing or the isolation technique, because the Cobe Spectra (Gambro BCT) machines are reported to cause activation of a small amount of platelets during collection.32 When platelet contamination is decreased by prestorage leukoreduction with a filter that removes platelets, the generation of sCD40L is decreased. These results are congruous with estimates that 95% of all human sCD40L is platelet derived,33 both in apheresis procedures and in the collection of whole blood for separation into components. Although these concentrations are not large, a significant amount may be infused into patients requiring massive blood support.34,35
Although sCD40L has the capacity to prime the PMN oxidase and to activate the microbicidal arsenal of PMNs adherent to HMVECs (ie, primed PMNs), other proinflammatory compounds also increase during the storage time of PCs, especially lipids,15 which have the capability to cause TRALI in susceptible hosts.11,36,37 Furthermore, sCD40L actually decreased or was unchanged in 4 of 12 patients with TRALI. Thus, determining the biologic significance of sCD40L in patients' plasma requires further study. Importantly, sCD40L may be rapidly internalized or it may become ligated to CD40, which resides on a number of cells in the human vasculature.38-40 Soluble CD40L could also serve as a cofactor in TRALI because of its ability to activate circulating monocytes or the vascular endothelium causing increases in the levels of pro-inflammatory chemokines.17 Moreover, though A-Plts are thought to be a purer platelet product than WB-Plts with respect to the increased rate of adverse events, sCD40L was increased in these components compared with WB-Plts and thus may have unexpected effects in the transfused host.41,42 A recent report revealed that sCD40L is associated with adverse events in patients requiring platelet transfusion (in press).43 Furthermore, sCD40L may have direct effects on the innate and adaptive immune system and may contribute to or cause TRALI and other adverse events in human patients, especially those requiring aggressive blood support for organ transplantation, a group with an unexplained increase in TRALI reactions in one series.3,44-48
In summary, sCD40L is a proinflammatory protein that accumulates during the storage of A-Plts and other cellular blood components. In PRBCs, it appears that leukocytes directly influence its accumulation in the plasma; however, in PCs, even those with minimal leukocyte contamination, sCD40L is released into the plasma in high concentrations. A-Plts have been implicated in TRALI,3,36,37,49 and injured patients with thrombocytopenia requiring platelet support are at risk for postinjury multiple organ failure, which invariably includes acute lung injury.50-52 Little research has focused on the mediators contained within platelet membranes or in storage granules—α-granules, dense bodies, or lysosomes—agents that may be responsible for proinflammatory and immunomodulatory effects in the transfused host.53 To date, the only effective method of removing proteins or polypeptides from the plasma fraction of cellular blood components is washing, which can be expensive and time consuming and is not suitable for all patient groups.29 Further work is required to optimize platelet support for patients at risk for transfusion-related inflammation or immunomodulation and to determine the biologic affects of native sCD40L with respect to its role in transfusion reactions, especially TRALI and its inherent ability to directly cause activation of the PMN microbicidal arsenal in vivo.
Prepublished online as Blood First Edition Paper, June 13, 2006; DOI 10.1182/blood-2006-04-017251.
Supported by Bonfils Blood Center, the National Heart, Lung and Blood Institute (grants HL59355-06 and HL078603), the National Institute of General Medical Sciences (grant P50 GM49222-12), and the United States Public Health Service (grants ES01347 and DE0113910).
All authors contributed significantly to this article. S.Y.K. performed most of the experiments at Bonfils Blood Center and wrote the article. M.R.K. performed some experiments and helped to write the article. J.M.H. performed some of the experiments in Rochester and provided substantive input for experimental design, data analyses, and manuscript preparation. N.B. provided the idea for the manuscript with J.M.H., and provided data analyses and helped to write the manuscript. L.K.B. obtained all samples in Edmonton (AB, Canada) and helped in manuscript preparation. R.P. provided expertise in CD40:CD40L interactions, provided important data analyses, and helped to write the manuscript. K.F.G. performed the modified sCD40L ELISA in Rochester. N.J.M. completed the microscopy. C.C.S. was instrumental in experimental design, data analyses, and the writing of the manuscript.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Daniel Ambruso (Bonfils Blood Center and the Department of Pediatrics, University of Colorado School of Medicine), Larry Dumont (Gambro BCT), and Deborah Dumont (Whatman) for providing data regarding the removal of platelets by the Hemasure r/LS leukoreduction filter.