Abstract
Recent evidence indicates that regulatory T cells (Tregs) play an important role in HIV infection. However, although the gastrointestinal mucosa is a key compartment in HIV disease, no data on mucosal Tregs in HIV infection are available. In this study, we compared the frequency of Tregs in duodenal mucosa and peripheral blood (PB) of 13 treatment-naive and 13 suppressively treated HIV-infected patients with that of 6 patients with norovirus infection and 12 healthy controls. Tregs were quantified by immunohistochemistry (CD3/FOXP3) and further characterized (CD25, CTLA-4, GITR) by immunohistochemistry, immunofluorescence, and fluorescence-activated cell sorting (FACS). Both the frequency and the absolute count of mucosal Tregs were highly increased in untreated HIV patients but were normal in treated HIV patients. In contrast, in peripheral blood of HIV patients, the absolute number of Tregs was not increased, and their frequency was only slightly elevated. In norovirus infection, frequency of mucosal Tregs in the CD4+ T-cell subset was not elevated. The high increase in count and frequency of mucosal Tregs seems to be a characteristic feature of untreated HIV infection, suggesting a significant contribution of Tregs to the pathogenesis of HIV disease. Their role may be 2-edged: attenuating HIV-induced immune hyperactivation while suppressing the immune response to HIV and mucosal pathogens.
Introduction
Recent studies indicate that regulatory T cells (Tregs) contribute to HIV-induced immune dysfunction.1-5 In chronic HIV infection, Tregs were shown to suppress HIV-specific CD4+ T-cell and CD8+ T-cell responses.1-3,5 However, whether or not the frequency of peripheral Tregs is increased in chronic HIV-infected individuals is controversial.1-6 Furthermore, although there is convincing evidence that Tregs do not exert their immunosuppressive action systemically but rather in a locally restricted manner at sites of antigen expression or pathogen replication,7,8 only one study examined Tregs in lymphoid tissue of HIV-infected patients. In this study, an increased tonsillar expression of molecular markers of Tregs was found.9 Data on Tregs in the gastrointestinal mucosa have not been reported so far, although the gastrointestinal tract has been identified as a key compartment of HIV infection.10-16 Harboring most of the body's lymphocytes, it is a compartment of high viral replication and pronounced CD4+ T-cell depletion.10-16 Furthermore, in AIDS patients, the gastrointestinal tract is extremely vulnerable to a great variety of opportunistic infections, although the mechanisms of the mucosal immune failure have not been fully elucidated.17
To gain further insight into the role of Tregs in the pathogenesis of HIV infection, we evaluated the number of Tregs in the duodenal mucosa and in the peripheral blood of HIV-infected patients. A prerequisite for this study was the capability to distinguish Tregs from other T cells residing in the intestinal mucosa. Fortunately, during the past several years a number of cell markers differentiating Tregs from other T-cell subsets have been discovered. Initially, the constitutively high expression of the α-chain of the IL-2 receptor, CD25, in the majority of Tregs was used for the identification and functional characterization of Tregs in rodents and humans.18,19 Since then it has been shown that CD25+ Tregs also constitutively express CTLA-4 18-20 and the glucocorticoid-induced TNF receptor-related gene, GITR.19,21 CD25, CTLA-4, and GITR represent receptor molecules, and there is rapidly growing experimental evidence for a close connection between signal transduction through these receptors and the function of Tregs.18,19 Still, although widely adopted as markers specific for Tregs, they can also be expressed by nonregulatory T cells upon activation. Therefore, it has been a great progress when a gene specifically controlling development and function of Tregs has been identified.22,23 This gene belonging to the forkhead transcription factor family and designated Foxp3 (or FOXP3 in humans) is constitutively expressed by CD25+CD4+ Tregs and required for their development. Furthermore, retroviral transfer of Foxp3 to nonregulatory CD25–CD4+ T cells converted them into Tregs expressing high levels of CD25, CTLA-4, and GITR and displaying suppressive activity in vitro as well as in vivo.22,23 Therefore, we used immunhistochemical detection of FOXP3 as a specific marker for Tregs18,19,22-25 and found significant accumulation of Tregs in the duodenal mucosa of treatment-naive HIV patients despite HIV-induced depletion of mucosal CD4+ T cells. The resulting high ratio of regulatory to nonregulatory CD4+ T cells within the intestinal mucosa of untreated HIV-infected patients is suggestive for a distinct contribution of mucosal Tregs to the HIV-induced mucosal immune defect.
Patients, materials, and methods
Subjects
Four groups of patients were included in the study: treatment-naive HIV-infected patients (n = 13), HIV-infected patients treated by highly active antiretroviral therapy (HAART, n = 13), patients with norovirus infection (n = 6), and patients without gastrointestinal pathology (“healthy controls,” n = 12). Clinical details of the study patients are provided in Table 1. HIV-infected patients underwent endoscopy for various symptoms related to the gastrointestinal tract such as nausea, abdominal pain, heartburn, and weight loss. Treatment-naive patients had never received antiretroviral therapy. In all treated patients, HIV replication had been suppressed below the limit of detection (quantitative polymerase chain reaction [PCR], 50 copies per milliliter; Roche Amplicor, Roche, Mannheim, Germany) by HAART for a minimum of 3 months (median time below limit of detection, 12 months; range, 3-67 months). Antiretroviral therapy consisted of a standard combination of 2 nucleoside reverse transcriptase inhibitors together with either a nonnucleoside reverse transcriptase inhibitor (n = 7) or a ritonavir-boosted protease inhibitor (n = 6). At the time of the endoscopy, active stage C disease was present in 2 treatment-naive (1 patient with toxoplasma encephalitis and 1 patient with candida esophagitis) and in 2 treated HIV-infected patients (candida esophagitis in both patients). Norovirus infection was clinically suspected according to Kaplan's criteria26 and confirmed by detection of viral RNA in stool samples by routine PCR. Biopsies obtained from 12 patients undergoing endoscopy for exploration of unexplained anemia served as “healthy controls.” Patients with pathological lesions of the duodenum and/or antrum as detected by endoscopic or histological examination (eg, Helicobacter pylori gastritis, mucosa-associated lymphoid tissue [MALT] lymphoma, Kaposi sarcoma) were excluded. In addition to these study groups, 4 HIV-infected patients were investigated serially at the beginning of antiretroviral therapy and again after more than 3 months of suppression of plasma viral load by HAART. Table 2 provides clinical details of these patients. The study was approved by the local ethics committee, and all patients agreed by written informed consent to study participation.
Isolation of cells
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized venous blood by density gradient centrifugation with Ficoll Paque (Amersham, Freiburg, Germany) in Leucosep-tubes (Greiner, Frickenhausen, Germany). Cells were either immediately stained for flow cytometry or kept frozen in fetal calf serum/10% DMSO in liquid nitrogen for later analysis. Duodenal lymphocytes were isolated as described previously.27 In short, biopsies were washed in sterile PBS, cut into small pieces, and incubated in 40 mL digestion medium (RPMI 1640; Invitrogen, Karlsruhe, Germany) supplemented with 10% FCS (Sigma, Taufkirchen, Germany), penicillin/streptomycin/amphotericin (Biochrom, Berlin, Germany), 0.5 mM β-mercaptoethanol (β-ME; Sigma), 0.2 mg/mL collagenase (Biochrom), 0.1 mg/mL DNase (Roche), and 0.1 mg/mL trypsin inhibitor (Sigma) for 3 hours at 37°C/5%CO2 on an orbital shaker. After passing the cell suspension through a stainless steel mesh and a 70-μm cell strainer (Becton Dickinson, Heidelberg, Germany), cells were washed twice with PBS/0.5% BSA (Sigma) and stained for flow cytometric analysis.
Flow cytometric analysis
Cells were incubated for 10 minutes at room temperature (RT) in 50 μL blocking buffer (PBS/0.5% BSA containing 2% Beriglobin [Behring, Hattersheim, Germany]). Indicated antibodies were then added in 50 μL PBS/0.5% BSA/0.02% NaN3 (PBA) for an additional 15 minutes. Optimal concentrations of staining antibodies were determined in independent experiments (not shown). Staining of FOXP3 was performed according to the manufacturer's protocol (eBioscience, San Diego, CA). Cells were washed and resuspended in PBA for fluorescence-activated cell sorting (FACS) analysis. Antibodies used in this study were CD4-FITC (MT310; Dako, Glostrup, Denmark), CD3-APC (UCHT-1; BD Pharmingen, Heidelberg, Germany), CD25-PE (ACT-1; Dako), CD8-PE, CD8-FITC (DK-25; Dako), Foxp3-PE, Foxp3-APC (PCH101; eBioscience), rat IgG2a-PE, rat IgG2a-APC (isotype controls), CD25-APC (2A3; BD Pharmingen), and CD4-PerCPCy5.5 (SK3; BD Pharmingen).
Acquisition of data was performed on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). Data were collected and analyzed with CellQuest software (Becton Dickinson, San Jose, CA). T-lymphocyte populations were gated based on forward and side scatter and expression of CD3. At least 10 000 CD4+ lymphocytes were acquired for analysis of FOXP3+ cells (peripheral blood).
Immunohistochemistry
For immunostaining, 4-μm sections were cut, deparaffinized, and subjected to a heat-induced epitope retrieval step before incubation with antibodies. Sections were immersed in sodium citrate buffer solutions at pH 6.0 and heated in a high-pressure cooker. The slides were rinsed in cool running water, washed in Tris-buffered saline (pH 7.4), and incubated with primary antibodies. The primary antibodies included monoclonal antibodies against CD4 (clone 1F6, dilution 1:25; Novocastra Laboratories, Newcastle upon Tyne, United Kingdom) and CD8 (C8/144B, 1:100; Dako). For detection, the alkaline phosphatase–antialkaline phosphatase complex (APAAP) method was used. Alkaline phosphatase was revealed by Fast Red as chromogen. For double immunoenzymatic labeling, slides were blocked using a peroxidase-blocking reagent (Dako) and incubated for 30 minutes with the goat polyclonal antibody against the C terminus of the FOXP3 protein (ab2481, dilution 1:50; Abcam, Cambridge, United Kingdom) followed by rabbit antigoat and the EnVision peroxidase kit (Dako). For comparison, consecutive sections were stained with a different commercially available anti-FOXP3 antibody (clone PCH101, 1:200; eBioscience) followed by rabbit antirat (Dako) and the EnVision peroxidase kit. Sections were then incubated for an additional 30 minutes with the second antibody against CD3 (UCHT1, 1:25; Dako), and for detection the APAAP method was used. For double labeling of FOXP3/CD25 or FOXP3/CD8, the anti-CD25 antibody (clone 4C9, 1:100; Novocastra) or anti-CD8 antibody was incubated for 30 minutes as second primary antibody and detected using biotinylated donkey antimouse antibody (1:200, Dianova; Hamburg, Germany) and streptavidin-AP (Dako). Tonsillar tissue with follicular hyperplasia served as positive controls displaying scattered T cells in the interfollicular area with nuclear expression of FOXP3. Negative controls were performed by omitting the primary antibodies. Positive cells were quantified in intestinal tissues per high-power field (1 hpf = 0.237 mm2), and 10 hpf was averaged in each case. Mononuclear cells located in lymphatic aggregations and intraepithelial lymphocytes were not taken into account. The frequencies determined therefore refer to lamina propria lymphocytes (LPLs). The frequency of FOXP3+ T cells per mucosal CD3+ T cells was determined by dividing the number of CD3+FOXP3+ LPLs by the number of mucosal CD3+ cells. The frequency of mucosal FOXP3+ T cells in the CD4+ T-cell subset was determined by dividing the number of CD3+FOXP3+ LPLs by the number of mucosal CD4+ cells. All immunohistochemical evaluations were performed in a blinded manner (ie, unaware of the patients'clinical data).
Immunofluorescence and confocal microscopy
For immunofluorescence staining, 1.5-μm sections were cut, deparaffinized, and subjected to a heat-induced epitope retrieval step (30 minutes, 100°C, microwave in sodium citrate buffer solutions at pH 6.0) before incubation with antibodies. Sections were washed with Tris-buffered saline (TBS)/0.5% human serum after each antibody. Primary antibodies, including anti-Foxp3 (PHC101; eBioscience), anti-CD25 (4C9; Novocastra), anti-HLADR (TAL1B5; Dako), anti-CD38 (SPC32; Novocastra), anti–CTLA-4 (R&D Systems, Wiesbaden, Germany), anti-GITR (R&D Systems), and anti-CD3 (AMS Biotechnology, Wiesbaden, Germany), were added in TBS (pH 7) containing 5% human serum and incubated either overnight at 4°C (FOXP3) or for 1 hour at RT. Sections were then incubated for 45 minutes at RT with secondary antibodies as indicated (Figure 2): biotinylated antirat antibody (Dako), digoxigenized (in-house) antirabbit antibody (Dianova), anti–goat Cy3 (Chemicon, Hampshire, United Kingdom), and anti–mouse Alexa 488 (Molecular Probes, Eugene, OR), followed by additional incubation as indicated with SA-Cy3 or SA-Cy2 (both Dianova) and antidigoxigenin (Roche) conjugated to Cy5 (kindly provided by Deutsches Rheumaforschungszentrum, Berlin, Germany). Negative controls were performed by omitting the primary antibodies. Sections were mounted in Fluoromont G (Biozol, Tübingen, Germany), and fluorescence images were taken with the Zeiss confocal microscope LSM510 (magnification × 63 or × 40; excitation wavelengths, 364, 488, and 543 nm).
Statistical analysis
Data were analyzed by means of the Kruskal-Wallis test. The Dunn multiple comparison test was used for post hoc analysis. P values less than .05 were considered significant.
Results
Characterization of lamina propria Tregs
For quantitative analysis, lamina propria Tregs were detected immunohistochemically by double immunoenzymatic labeling as LPLs expressing both CD3 and the transcription factor FOXP3 using 2 different commercially available antibodies against FOXP3 that yielded basically identical staining results.28 To further characterize these cells, we investigated coexpression of FOXP3 with CD25 in mononuclear cells obtained from the duodenal mucosa and the peripheral blood using flow cytometry. Due to the limited number of duodenal biopsies available, duodenal lymphocytes were analyzed only in 4 HIV patients (1 treated, 3 untreated). In all samples analyzed, we detected a distinct population of FOXP3+CD25high cells within the CD4+ T-cell subset in both mononuclear cells derived from the duodenal mucosa as well as from the peripheral blood (Figure 1). Coexpression of FOXP3 and CD25 in LPLs was confirmed by immunohistochemistry and immunofluorescence. In duodenal mucosa sections from all patient groups, FOXP3+ LPLs also expressed CD25 (Figure 2B; Table 3). In addition, most (60% to 90%) cells expressing FOXP3 coexpressed CTLA-4 and GITR (Figure 2A,C; Table 3). Although the gastrointestinal mucosa is known to be abundant with activated T cells, we found few LPLs positive for CD25 but not for FOXP3, indicating that our immunohistochemistry and immunofluorescence protocols predominantly detected the CD25high fraction, whereas activated T cells with intermediate expression of CD25 were not stained (Figure 3). Furthermore, we did not observe coexpression of FOXP3 and markers for T-cell activation, like CD38 and HLA-DR (Figure 2D,E; Table 3). Therefore, significant dilution of mucosal FOXP3+ cells by nonregulatory activated T cells was excluded. In addition, we did not find significant percentages of FOXP3+ cells in non-CD4 cells in the mucosa (0% by immunohistochemistry; 0.15% ± 0.07% by FACS) as well as in the peripheral blood (0.42% ± 0.3% by FACS). Taken together, using different methods we obtained clear evidence that the FOXP3+ cells detected by our staining protocol represent a regulatory phenotype without signs of activation.
Representative flow cytometric analysis of FOXP3+ cells. Staining for FOXP3+ cells on peripheral blood lymphocytes (A,B,D,E) or duodenal lymphocytes (C,F) obtained from an untreated HIV-infected patient. Cells were gated for CD4+ lymphocytes (A-C) or for lymphocytes in the forward-sideward scatter (D-F). Numbers indicate the frequency of FOXP3+CD25high cells (A-C) or the frequency of cells in each quadrant (D-F).
Representative flow cytometric analysis of FOXP3+ cells. Staining for FOXP3+ cells on peripheral blood lymphocytes (A,B,D,E) or duodenal lymphocytes (C,F) obtained from an untreated HIV-infected patient. Cells were gated for CD4+ lymphocytes (A-C) or for lymphocytes in the forward-sideward scatter (D-F). Numbers indicate the frequency of FOXP3+CD25high cells (A-C) or the frequency of cells in each quadrant (D-F).
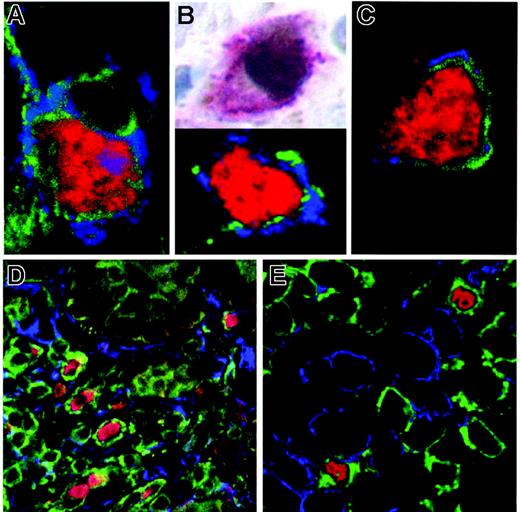
Characterization of mucosal FOXP3+ cells. Counterstaining of FOXP3 (red; detection with SA-Cy2 or SA-Cy3), CD3 (green; anti–Dig-Cy5) with surface markers of Tregs (blue; anti–goat Cy3) CTLA-4 (A), GITR(C), and CD25 (blue; anti–mouse Alexa 488) by immunofluorescence (B, bottom panel) or of FOXP3 (brown; immunoperoxide) with CD25 (red; APAAP) by immunohistochemistry (B, top panel) revealed clear coexpression. On the other hand, counterstaining of FOXP3 (red), CD3 (green), and T-lymphocyte activation markers (blue; anti–mouse Alexa488) HLA-DR (D) and CD38 (E) did not detect any cells positive for both markers. Depicted are representative sections from duodenal biopsies of HIV-infected patients. Original magnification ×600 (A-C) and ×400 (D-E). Microscope: Olympus AX70 (Olympus, Hamburg, Germany). Numerical aperture of objective lenses: ×40, 0.95 mm; ×60, 1.40. Camera: JVC KY-F70 (JVC, Yokohama, Japan). Acquisition software: DISKUS (Carl H. Hilgers, Königswinter, Germany). Software used for image processing: Adobe Photoshop 7.0 (Adobe, San Jose, CA).
Characterization of mucosal FOXP3+ cells. Counterstaining of FOXP3 (red; detection with SA-Cy2 or SA-Cy3), CD3 (green; anti–Dig-Cy5) with surface markers of Tregs (blue; anti–goat Cy3) CTLA-4 (A), GITR(C), and CD25 (blue; anti–mouse Alexa 488) by immunofluorescence (B, bottom panel) or of FOXP3 (brown; immunoperoxide) with CD25 (red; APAAP) by immunohistochemistry (B, top panel) revealed clear coexpression. On the other hand, counterstaining of FOXP3 (red), CD3 (green), and T-lymphocyte activation markers (blue; anti–mouse Alexa488) HLA-DR (D) and CD38 (E) did not detect any cells positive for both markers. Depicted are representative sections from duodenal biopsies of HIV-infected patients. Original magnification ×600 (A-C) and ×400 (D-E). Microscope: Olympus AX70 (Olympus, Hamburg, Germany). Numerical aperture of objective lenses: ×40, 0.95 mm; ×60, 1.40. Camera: JVC KY-F70 (JVC, Yokohama, Japan). Acquisition software: DISKUS (Carl H. Hilgers, Königswinter, Germany). Software used for image processing: Adobe Photoshop 7.0 (Adobe, San Jose, CA).
Absolute number and frequency of mucosal and peripheral blood Tregs in HIV-infected and control patients
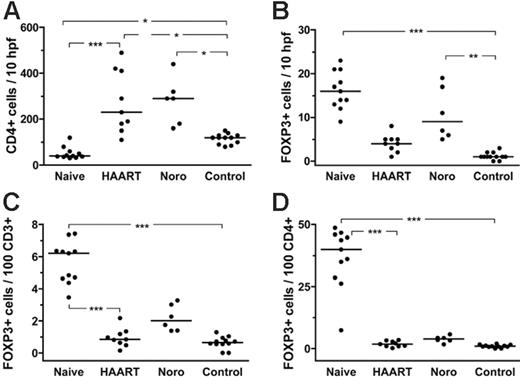
In treatment-naive HIV-infected patients, we found a high absolute number (cells per high-power field; Figures 3 and 4B) and also a high frequency (cells per T-cell subset; Figure 4C-D) of mucosal Tregs (Figures 3, 4). Within the CD4+ T-cell subset, the median percentage of Tregs was tremendously increased to 40.0% of lamina propria CD4+ T cells, compared with only 1.2% in healthy controls (P < .001; Figure 4D). The total number of mucosal CD4+ T cells as analyzed by immunohistochemistry was significantly reduced in untreated HIV-infected patients compared with healthy controls (Figure 4A), and thus the high frequency of Tregs within the mucosal CD4+ T cells subset can be attributed to both an increased absolute number of mucosal Tregs as well as to loss of mucosal CD4+ T cells other than Tregs (Figures 3 and 4D). In contrast, FACS analysis of Tregs in the peripheral blood revealed only a minor increase in the frequency of Tregs (Tregs per CD4+ T cells) in untreated HIV-infected patients (Figure 5A) whereas their absolute number (Tregs per microliter of blood) was not different from that of healthy controls (Figure 5B). In treated HIV-infected patients, on the other hand, the absolute number as well as the frequency of mucosal and peripheral Tregs were not significantly different from those found in healthy controls (Figures 3, 4, 5).
Treatment-induced changes of mucosal Tregs were investigated longitudinally in 4 HIV-infected individuals (Table 2; Figure 6). In these patients, duodenal biopsies were obtained first prior to (patients A, C, D) or shortly after (patient B) initiation of antiretroviral therapy and later when HIV replication had been suppressed by HAART for at least 3 months. In these patients, too, the absolute number and the frequency of mucosal Tregs were increased in the pre-HAART biopsies but returned to the level of healthy controls after suppression of viral replication (Figure 6), confirming the results of the unpaired groups of untreated and treated HIV-infected patients.
In patients with norovirus infection, the absolute number of mucosal Tregs was also increased compared with healthy controls, although not to the extent seen in untreated HIV-infected patients (Figures 3, 4). Furthermore, due to a concomitant increase of mucosal CD4+ T cells, the frequency of mucosal Tregs in the CD4+ T-cell subset was only marginally elevated compared with healthy controls (Figure 4). Therefore, the remarkable increase in the frequency of mucosal Tregs in the CD4+ T-cell subset seems to be a characteristic feature of untreated HIV infection.
Discussion
The gastrointestinal tract is involved in central steps of HIV-host interaction as well as in many clinical manifestations secondary to HIV-induced immunodeficiency.17 However, although an important role of Tregs in the immune pathogenesis of HIV infection is increasingly acknowledged,1-6,29,30 data on mucosal Tregs have not been reported so far.
In this study, data on the local frequency of Tregs in the gastrointestinal mucosa of HIV-infected patients are presented for the first time. We found a high absolute number and high frequencies of mucosal Tregs in treatment-naive HIV-infected patients. In these patients, more than one third of mucosal CD4+ T cells were phenotypically Tregs. This extremely high ratio seems to be a finding specific for HIV infection, because in patients infected with norovirus (data from this study) or H pylori31 —conditions that may serve as examples for acute or chronic upper gastrointestinal tract infections other than HIV—the frequency of mucosal Tregs in the CD4+ T-cell subset was only marginally elevated. Because the norovirus-infected patients were older than the untreated HIV-infected patients investigated, one might argue that the low number of mucosal Tregs in norovirus-infected patients as compared with untreated HIV-infected patients could be related to age. However, studies on peripheral T cells indicate that aging is associated with increased rather than decreased numbers of (peripheral) Tregs,32 and in our small groups of patients, we did not find any age-associated changes of mucosal Tregs in healthy controls or in HIV-infected patients (data not shown).
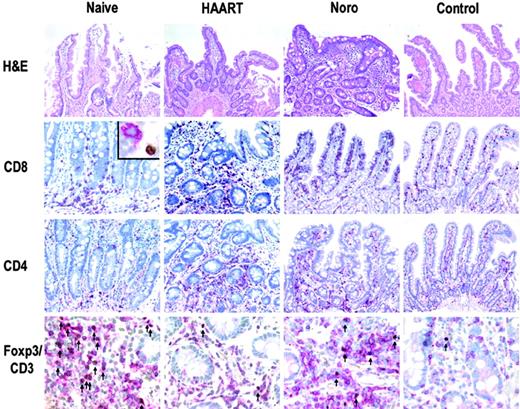
Representative immunohistochemical analysis of T-cell subsets in duodenal mucosa. Tregs (arrows, bottom row), identified by double immunoenzymatic labeling for CD3 (red, membranous) and FOXP3 (brown, nuclear), showed a dramatic increase in untreated HIV-infected patients. Inset: CD8+ T cell (red) without expression of FOXP3 (brown). Original magnification ×200, ×400 (bottom row), and × 600 (inset). Microscope: Olympus AX70. Numerical aperture of objective lenses: ×20, 0.70 mm; ×40, 0.95; ×60, 1.40. Stains: hematoxylin and eosin (top panel only); APAAP and immunoperoxide. Camera: JVC KY-F70 (JVC). Acquisition software: DISKUS. Software used for image processing: Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Representative immunohistochemical analysis of T-cell subsets in duodenal mucosa. Tregs (arrows, bottom row), identified by double immunoenzymatic labeling for CD3 (red, membranous) and FOXP3 (brown, nuclear), showed a dramatic increase in untreated HIV-infected patients. Inset: CD8+ T cell (red) without expression of FOXP3 (brown). Original magnification ×200, ×400 (bottom row), and × 600 (inset). Microscope: Olympus AX70. Numerical aperture of objective lenses: ×20, 0.70 mm; ×40, 0.95; ×60, 1.40. Stains: hematoxylin and eosin (top panel only); APAAP and immunoperoxide. Camera: JVC KY-F70 (JVC). Acquisition software: DISKUS. Software used for image processing: Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
Differing from the mucosa, the frequency of Tregs in the peripheral blood was not significantly increased in treatment-naive HIV-infected patients compared with treated patients and only slightly increased compared with healthy controls. In previous studies some investigators also observed a small increase of peripheral Tregs in untreated but not in treated HIV-infected patients,4 whereas others found evidence for normal or reduced numbers of peripheral Tregs in HIV infection.1,9 These differences most probably reflect the different methods used for quantification of Tregs (FACS for CD4+CD25high,3,4,6 FACS for CD4+CD25+,1 reverse transcriptase–PCR for FOXP3 transcripts6,9 ) as well as the heterogeneity of the patient population investigated (eg, treatment status of patients unspecified3 or inclusion of treated6 or untreated1 patients only). Apart from these considerations, our results clearly indicate that in untreated HIV-infected patients there is a striking difference in the distribution of Tregs with local accumulation at tissue sites of HIV replication like the tonsils9 and even more the gastrointestinal mucosa (data from this study) but normal1,6 or only slightly increased3,4 numbers of Tregs in the periphery.
Frequency of mucosal FOXP3+ Tregs in treatment-naive and treated HIV-infected patients, patients with norovirus infection, and in healthy controls. (A) Absolute number of mucosal CD4+ T cells per 10 hpf. (B) Absolute number of mucosal FOXP3+ Tregs per 10 hpf. (C) Frequency of lamina propria Tregs in relation to lamina propria CD3+ T cells and (D) to lamina propria CD4+ T cells. The horizontal lines denote the median values of each group. *P < .05, **P < .01, ***P < .001.
Frequency of mucosal FOXP3+ Tregs in treatment-naive and treated HIV-infected patients, patients with norovirus infection, and in healthy controls. (A) Absolute number of mucosal CD4+ T cells per 10 hpf. (B) Absolute number of mucosal FOXP3+ Tregs per 10 hpf. (C) Frequency of lamina propria Tregs in relation to lamina propria CD3+ T cells and (D) to lamina propria CD4+ T cells. The horizontal lines denote the median values of each group. *P < .05, **P < .01, ***P < .001.
For characterization of mucosal Tregs we detected LPLs positive for the cell markers currently regarded as the most specific markers for Tregs: FOXP3, CD25, CTLA-4, and GITR.18,19 Whereas all of these markers are considered to be expressed by Tregs, they might also be expressed by activated T lymphocytes. However, using confocal analysis we did not observe any coexpression of activation markers (CD38, HLA-DR) on FOXP3+ duodenal lymphocytes. Therefore, it seems unlikely that the FOXP3+CD3+ cells detected in the lamina propria represent activated T cells. Furthermore, flow cytometry revealed a distinct subset of FOXP3+CD4+ T lymphocytes with high expression of CD25 in both peripheral blood lymphocytes (PBLs) as well as LPLs. Thus, using different methods we obtained clear evidence for a specific and accurate detection of lamina propria Tregs by our immunohistochemistry.
Regulatory T cells in peripheral blood. (A) Frequency of FOXP3+CD25high cells (gated on CD4+ cells) determined by FACS. (B) Absolute number (given as counts per microliter of blood) of CD4+FOXP3+CD25high cells determined by FACS. The horizontal lines denote the median values of each group. ***P < .001.
Regulatory T cells in peripheral blood. (A) Frequency of FOXP3+CD25high cells (gated on CD4+ cells) determined by FACS. (B) Absolute number (given as counts per microliter of blood) of CD4+FOXP3+CD25high cells determined by FACS. The horizontal lines denote the median values of each group. ***P < .001.
Longitudinal analysis of LPLs in 4 HIV-infected patients at the beginning (pre-ART) and during HAART. (A) CD4+ LPLs per 10 hpf, (B) FOXP3+ LPLs per 10 hpf, and (C) FOXP3+ T cells in percent of CD4+ LPLs. Clinical characteristics of the patients are provided in Table 2. Patient B (○) had already been treated by HAART at the first endoscopy for 8 weeks, while the other patients were initially treatment naive.
Longitudinal analysis of LPLs in 4 HIV-infected patients at the beginning (pre-ART) and during HAART. (A) CD4+ LPLs per 10 hpf, (B) FOXP3+ LPLs per 10 hpf, and (C) FOXP3+ T cells in percent of CD4+ LPLs. Clinical characteristics of the patients are provided in Table 2. Patient B (○) had already been treated by HAART at the first endoscopy for 8 weeks, while the other patients were initially treatment naive.
Although the tremendous increase of mucosal Tregs in the CD4 cell subset observed in untreated HIV-infected patients might partly result from depletion of nonregulatory CD4+ T cells, the increased absolute number of mucosal Tregs clearly indicates a process of active recruitment into and/or local expansion within the duodenal mucosa. In the peripheral blood of these patients, on the other hand, the frequency but not the absolute number of Tregs was increased, indicating depletion of nonregulatory CD4+ T cells rather than active accumulation of Tregs in the periphery. In our small groups of HIV-infected patients, we did not find a correlation between the frequency of mucosal Tregs and general immune status of the patients as reflected by the number of their peripheral CD4+ T cells. Furthermore, possibly due to the small number of patients tested, plasma viral load also did not correlate with the number of mucosal Tregs (data not shown). However, after suppression of viral replication by HAART, the frequency of mucosal Tregs returned to the level of healthy controls. Therefore, one may hypothesize that mucosal accumulation of Tregs is linked to HIV replication. This scenario would fit well into a scheme of Tregs cell expansion under circumstances of antigen persistence as has been described in other infectious conditions, like H pylori,31 Leishmania,33 or hepatitis C virus infection.34 However, considering our limited knowledge of the specificity, generation, and migration of mucosal Tregs, future investigations will have to test this hypothesis.
As to the function of mucosal Tregs, immune suppressive action has been demonstrated in lamina propria Tregs obtained from colonic biopsies.35 With a much smaller number of biopsies obtainable from the duodenum, studies on duodenal Tregs function were precluded by low cell numbers. However, the cell markers used for identification of mucosal Tregs are closely linked to the suppressive function of these cells. Therefore, indirect evidence indicates that the mucosal cells phenotypically characterized as Tregs also exert regulatory function, although this hypothesis can be proved only by functional studies.
There is strong experimental evidence that the inhibitory action of Tregs on effector lymphocytes is at least partly mediated by direct cell contact.20,36,37 Consequently, the intensity of their suppressive action is thought to correlate with the local ratio of regulatory to nonregulatory T cells.18,20,36,37 Therefore, the high frequency of mucosal Tregs in treatment-naive HIV-infected patients carries important implications for the immune pathogenesis of HIV infection. For example, as shown in a primate model, Tregs slowed SIV replication and disease progression possibly by counteracting the chronic T-cell hyperactivation characteristic for SIV and HIV infection,38 and in untreated HIV-infected patients the number of peripheral Tregs cells inversely correlated with markers of immune activation.29 Because chronic immune activation in HIV infection is considered a prominent cause for CD4+ T-cell depletion and disease progression,39-42 expansion of Tregs in lymphoid tissue like the gastrointestinal tract could be interpreted as a protective countermechanism trying to attenuate the HIV-induced chronic immune activation.
On the other hand, suppression of HIV-specific T-cell responses by Tregs may also be harmful to the host, because this may contribute to the anergy of the immune system to HIV.1-4 Studies in infection models described significant immunosuppressive activity of Tregs for antigen-specific T-cell responses against pathogens most relevant in the context of HIV infection, like Candida, Leishmania, Pneumocystis carinii, and herpes simplex virus.43 Therefore, the high susceptibility to gastrointestinal opportunistic infections in untreated HIV-infected patients could be due not only to loss of mucosal CD4+ T cells but also to the high number of mucosal Tregs in the gastrointestinal tract.
In summary, our data suggest that active HIV replication induces accumulation of Tregs in the gastrointestinal tract. The accumulation of Tregs at tissue sites of HIV replication may reflect an attempt to slow disease progression by suppression of the HIV-induced T-cell hyperactivation but may also increase the susceptibility of the gastrointestinal tract to opportunistic infections.
Prepublished online as Blood First Edition Paper, May 25, 2006; DOI 10.1182/blood-2006-04-016923.
Supported by grants from the German Research Foundation (grants DFG KFO 104 and SFB633) and from the German Federal Department for Research and Education (Competence Network HIV/AIDS 01 K I 0501).
H.-J. E., C. L., and D. K. contributed equally to this study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The excellent technical assistance of Simone Spiekermann and the expert advice of Jan Richter are gratefully acknowledged.