Abstract
The human genetic disease X-linked lymphoproliferative disease (XLP), which is caused by mutations in SH2D1A/SAP that encode SLAM-associated protein (SAP), is characterized by an inability to control Epstein-Barr virus (EBV) and hypogammaglobulinemia. It is unclear which aspects of XLP disease are specific to herpesvirus infection and which reflect general immunologic functions performed by SAP. We examined SAP– mice during a chronic LCMV infection, specifically to address the following question: Which SAP deficiency immunologic problems are general, and which are EBV specific? Illness, weight loss, and prolonged viral replication were much more severe in SAP– mice. Aggressive immunopathology was observed. This inability to control chronic LCMV was associated with both CD8 T-cell and B-cell response defects. Importantly, we demonstrate that SAP– CD8 T cells are the primary cause of the immunopathology and clinical illness, because depletion of CD8 T cells blocked disease. This is the first direct demonstration of SAP– CD8 T-cell–mediated immunopathology, confirming 30 years of XLP clinical observations and indirect experimentation. In addition, germinal center formation was extremely defective in chronically infected SAP– animals, and hypogammaglobulinemia was observed. These findings in a chronic viral infection mouse model recapitulate key features of human XLP and clarify SAP's critical role regulating both cellular and humoral immunity.
Introduction
X-linked lymphoproliferative disease (XLP) is a rare human genetic immunodeficiency. The disease is usually fatal during childhood and is associated with uncontrolled infections, particularly Epstein-Barr virus (EBV), but also measles virus, Neisseria meningitidis, pneumonia, and vaccinia virus among others. In addition, XLP is frequently characterized by progressive hypogammaglobulinemia and B-cell lymphomas. SH2D1A/SAP, which encodes SLAM-associated protein (SAP), was identified in 1998 as the genetic locus responsible for XLP.1-3 SAP is a small SH2-domain protein expressed in CD4 T cells, CD8 T cells, natural killer (NK) cells, NKT cells, and some B cells.3-6 SAP is now known to play a role in an impressive array of lymphocyte functions,7-9 including T-cell proliferation,10-13 inhibition of CD8 and CD4 T-cell IFNγ production,10,11,14,15 CD4 T-cell TH1/TH2 polarization,10,11,16,17 activation and inhibition of NK cell killing,18-20 cytotoxic CD8 T-cell activity,21-23 NKT-cell development,4,24,25 and germinal center formation and the generation of long-term humoral immunity.12,26,27
EBV infections are fatal in approximately half of SAP-deficient patients during childhood. Fulminant infectious mononucleosis is characterized by large numbers of EBV-infected B cells, multiorgan lymphocytic infiltrates, and extensive and rapid destruction of liver and bone marrow, though some XLP patients die from more prolonged EBV sequelae. XLP can also be characterized by severe difficulties with other viral and bacterial infections and chronic ailments related to infections.28-32 Dysgammaglobulinemia in XLP patients is observed both in the presence and absence of EBV infection33 and is generally observed as a progressive hypogammaglobulinemia with reduction in most IgG subclasses.34-36
SAP interacts with the cytoplasmic tail of SLAM/CD150.3 SAP is also able to associate with the cytoplasmic tail of most members of the SLAM family of receptors (SLAM, CD244, CD229, CD84, and Ly108/NTB-A).3,7,8,20,37-40 SAP binds to a tyrosine-based signaling motif termed the immunoreceptor tyrosine-based switch motif (ITSM),39 related to ITAM and ITIM motifs.41 Much of the signaling mediated by SAP in T lymphocytes appears to be mediated by recruitment of the protein tyrosine kinase FynT to SLAM or SLAM family receptors via a noncanonical SH3-domain interaction.8,14,16,17,42,43
Many connections remain unclear between the molecular and cellular biology of SAP function and the in vivo immunologic failures leading to XLP clinical disease. Of particular interest, it is unclear which aspects of XLP disease are specific to herpesvirus infection and which reflect general T-lymphocyte functions regulated by SAP. There is obviously a strong link between SAP deficiency and lethal Epstein-Barr virus (EBV) infection, and to understand that linkage it is critical to dissect the components of SAP immunobiology that are directly related to EBV pathogenesis versus those components that are more broadly involved in adaptive immune responses to infectious diseases. We show here that SAP– mice exhibit severe illness compared with wild-type mice during a chronic lymphocytic choriomeningitis virus (LCMV) infection, an RNA virus completely unrelated to EBV. Aggressive immunopathology was observed. Importantly, we demonstrate that CD8 T cells are the primary cause of the immunopathology and clinical illness observed in SAP– mice chronically infected with LCMV. This is the first direct demonstration of SAP– CD8 T-cell–mediated immunopathology, validating 30 years of clinical observations and indirect experimentation. In addition, germinal center formation was extremely defective in the chronically infected SAP– animals, and stark hypogammaglobulinemia was observed. These findings in a chronic viral infection mouse model recapitulate key features of human XLP—namely, severe CD8 T-cell–mediated immunopathology and hypogammaglobulinemia— and clarify SAP's critical role in the normal regulation of both cellular and humoral immune responses to viral infections.
Materials and methods
Mice
C57BL/6J (B6) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). SAP– mice backcrossed 7 generations to B6 were kindly provided by Dr Pamela Schwartzberg (National Institutes of Health, Bethesda, MD)10 and then bred at La Jolla Institute for Allergy and Immunology (LIAI). Genotyping was done as described.10 For chronic LCMVcl13 infection experiments, SAP– females were bred with B6 males to obtain F1 SAP– males, and B6 females were bred with SAP– males to obtain F1 SAP+ males. SAP– and SAP+ males from the outcross were used in the chronic infection experiments as near littermates. Experiments in Figures 6 and 7 were done using generation-10 backcrossed SAP– males matched with B6 males, and comparable results to littermate controls were seen. Five-week-old mice were used for LCMVcl13 experiments. Six- to 17-week-old mice were used for LCMVarm experiments. Infected and naive mice were provided identical diets. Mice in all groups were sex and age matched.
Viruses and infections
Plaque-forming units (PFU) of viral stocks, and serum and tissue samples from infected mice, were determined by 5-day plaque assay on VeroE6 cells (LCMVarm) or Vero cells (LCMVcl13).44
Plaque-purified clones of the Armstrong strain of LCMV (LCMVarm) were propagated in BHK-21 cells (American Type Culture Collection [ATCC], Manassas, VA)44 and tested for biologic activity in vitro and in vivo. A second passage stock of subclone SC3 (LCMVarm-sc3) was used for all LCMVarm experiments. For acute infections, mice received 1 × 105 PFU LCMVarm in a volume of 0.5 mL (suspended in RPMI 1640) by intraperitoneal inoculation.
Plaque-purified LCMV clone 13 (LCMVcl13) clones were propagated in BHK-21 cells44 and tested for biologic activity in vitro and in vivo. A second passage stock of subclone SC9 (LCMVcl13-sc9) was used for all LCMVcl13 experiments shown. To establish chronic infections, 5-week-old mice received 2 × 106 PFU LCMVcl13 by intravenous inoculation via either tail vein injection (0.5 mL) or retro-orbital injection (0.2 mL). Experiments in Figure 7 were done with a higher-dose intravenous infection, 4 × 106 PFU LCMVcl13, which causes more rapid disease. CD8 T-cell depletions were done at day –1 by intraperitoneal injection of 100 μL YTS-169 ascites (gift from Charles Surh, Scripps Research Institute, San Diego, CA) diluted to 500 μL in DMEM. Control experiments were performed to confirm that 100 μL YTS-169 completely depleted CD8 T cells (S.C., unpublished data, September 2005). A third experiment was done with anti-CD8 monoclonal antibody (mAb) clone Lyt2.43 purified from hybridoma culture supernatant, with comparable results. Rat IgG (Sigma, St Louis, MO) intraperitoneal negative control had no effect in multiple experiments (data not shown).
Cell and tissue preparation
Single cell suspensions of spleen, lymph, and bone marrow were prepared by standard techniques. For histology, spleen specimens were fixed overnight in 4% formalin, mounted in paraffin, and cut into 10-μm sections before hematoxylin and eosin counterstaining. Photographs were taken using a Nikon Optiphot microscope (Nikon, Melville, NY) with a 100×/0.2 NA air objective, equipped with a DVC 1310 digital camera (DVC, Austin, TX) attachment and C-View 2.2 acquisition software (DVC). Additional image optimization was done using Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA).
LCMV viral load QPCR
A detailed version of this assay will be published elsewhere (M.M.M. and S.C., unpublished data). Briefly, RNA was isolated from 50 μL serum or 10 to 75 mg tissue homogenate using RNAqueous (Ambion, Austin, TX). RNA was used in a 20-μL cDNA reaction with SuperScript III Reverse Transcriptase (Invitrogen, Carlsbad, CA) and a gene-specific primer. Complementary DNA was then used as template for a 25-μL quantitative real-time polymerase chain reaction (QPCR) on a GeneAmp 5700 (ABI, Redwood City, CA). A standard curve was generated using linearized pSG5-GP plasmid,45 a gift from Dr Juan Carlos de la Torre (Scripps Research Institute). All QPCR samples were run in duplicate. Standard curves were log-linear over more than a 105 range with high R2 values (about 98%). Multiple concurrent samples from uninfected mice were run as negative controls at all time points.
Plasma cell ELISPOT
Immunoglobulin ELISAs
Anti-LCMV antibody was measured by enzyme-linked immunosorbent assays (ELISAs) using sonicated cell lysate from LCMV-infected BHK-21 cells as capture antigen. Ninety-six–well Polysorp microtiter plates (NUNC, Rochester, NY) were coated overnight with lysate in PBS and then UV inactivated (300 mJ in Stratalinker 1800; Stratagene, La Jolla, CA). All samples were run in duplicate. HRP-conjugated goat anti–mouse immunoglobulin secondary antibodies directed against IgGγ or the isotype or IgG subclass of interest (Caltag Laboratories, Burlingame, CA) were used.
Duplicate 2-fold serial dilutions of mAb 1-1.3 were run in each LCMV ELISA as a calibrated standard. Mouse mAb 1-1.3 directed against LCMV nucleoprotein (NP) was obtained from Dr Michael Buchmeier (Scripps Research Institute).47 Absolute concentration of stock mAb 1-1.3 was determined by total IgGγ ELISA, titrating against a 0.5 mg/mL IgG2a standard antibody (Southern Biotechnology, Birmingham, AL). Standard curve R2 regularly exceeded 0.98, and the sensitivity was 3 ng/mL in all assays.
Total immunoglobulin was measured by quantitative ELISA using goat anti–mouse IgG+M+A (Caltag Laboratories) (6.25 μL/10 mL) as the capture antibody. Ninety-six–well Maxisorp microtiter plates (NUNC) were coated overnight and then used as described above. IgG standard curves were generated for each experiment (Southern Biotechnology).
Intracellular cytokine staining
A total of 1 × 106 cells were cultured in the absence or presence of the indicated peptide and brefeldin A for 5 to 6 hours at 37°C. H-2Db or H-2Kb restricted epitopes were used at 0.2 μg/mL and I-Ab restricted epitopes at 2 μg/mL. Following staining for surface antigens, cells were fixed and permeabilized with 2% (wt/vol) paraformaldehyde (PFA) and 0.1% saponin for 15 minutes and then stained for the intracellular cytokine of interest in the presence of 0.1% saponin and 2% NCS for 30 minutes. Cells were washed and then fixed in 2% ultrapure formaldehyde (Polysciences, Warrington, PA).
Flow cytometry analysis
Staining for flow cytometry used mAbs to CD4, CD8, CD3, B220, CD44, CD62L, IFNγ, TNF, and IL-2 (eBioscience San Diego, CA); polyclonal goat anti–mouse IgG (Caltag Laboratories); Fas and CD138 (BD PharMingen, San Diego, CA); and FITC-labeled PNA (Vector Laboratories, Burlingame, CA). Major histocompatibility complex (MHC) I tetramer of Db loaded with LCMV GP33-41 was kindly provided by Dr Hilde Cheroutre (LIAI). Cells were fixed in 2% ultrapure formaldehyde (Polysciences) prior to acquisition. Flow cytometry samples were acquired on a FACSCalibur instrument (Becton Dickinson, San Jose, CA) and analyzed using FlowJo software (TreeStar, San Carlos, CA).
Statistical analysis
Tests were performed using Prism 4.0 (GraphPad, San Diego, CA). Statistics were done using a 2-tailed, unpaired t test with 95% confidence bounds unless otherwise indicated. Data involving multiple time points were analyzed by 2-way analysis of variance (ANOVA). Error bars are ± 1 SEM unless otherwise indicated. Arithmetic means were used for all analyses except viral load data, where log10 scales and geometric means were used.
Results
Exacerbated clinical disease and increased viral loads in the absence of SAP
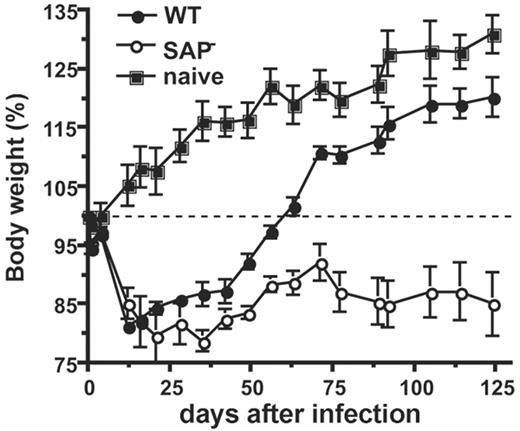
Infection with LCMVcl13 results in a persistent infection characterized by high viremia and high viral loads in numerous tissues (including liver, spleen, and kidney) for more than 1 month, followed by a period where viral replication is slowly brought under control and limited to persistent replication in the several tissues, including kidney and salivary gland.44,48-51 LCMVcl13 infection of wild-type mice results in substantial decline in body weight during the first 2 weeks, followed by a slow recovery of weight and overall health during a period of 1 to 3 months after infection, with weight gain becoming accelerated once viremia is substantially controlled (Figure 1). Clinical disease and viral clearance were examined at multiple time points in LCMVcl13-infected SAP– mice and wild-type controls. Wild-type mice began to slowly regain weight by 1 month after infection, but SAP– mice remained at their nadir (Figure 1). Body weights were significantly worse in SAP– than wild-type mice by 1 month after infection (P < .005). SAP– mice were also visibly more ill than the wild-type mice, as assessed by observation of ruffled fur and lethargy. Wild-type mice began to rapidly regain normal weight at approximately day 45 after infection, recovering 100% of initial body weight by days 60 to 75, and approached the weight of naive control mice by approximately 4 months after infection (Figure 1). In stark contrast, SAP– mice never recovered to their initial body weight (12 of 13 mice, less than 100% initial body weight at 4 months after infection; P < .001, 2-tailed Fisher test). Due to this failure to regain health, SAP-deficient LCMVcl13-infected mice were a dramatic 32% lower in weight than LCMVcl13-infected wild-type mice 4 months after infection (P < .002) (Figure 1). In addition, SAP– mice remained visibly ill.
Severe illness in LCMVcl13-infected SAP– mice. Body weight was tracked for SAP– (○) and SAP+ mice (•) infected with LCMVcl13, versus uninfected controls (▪). Data are presented as percentage of body weight on day 0 of infection. More severe illness was observed in SAP– mice. Three months after infection, SAP– mice weighed 30% less than wild-type mice (P < .002). SAP–, n = 8; wild-type B6 (WT), n = 3. Data are representative of 3 independent experiments.
Severe illness in LCMVcl13-infected SAP– mice. Body weight was tracked for SAP– (○) and SAP+ mice (•) infected with LCMVcl13, versus uninfected controls (▪). Data are presented as percentage of body weight on day 0 of infection. More severe illness was observed in SAP– mice. Three months after infection, SAP– mice weighed 30% less than wild-type mice (P < .002). SAP–, n = 8; wild-type B6 (WT), n = 3. Data are representative of 3 independent experiments.
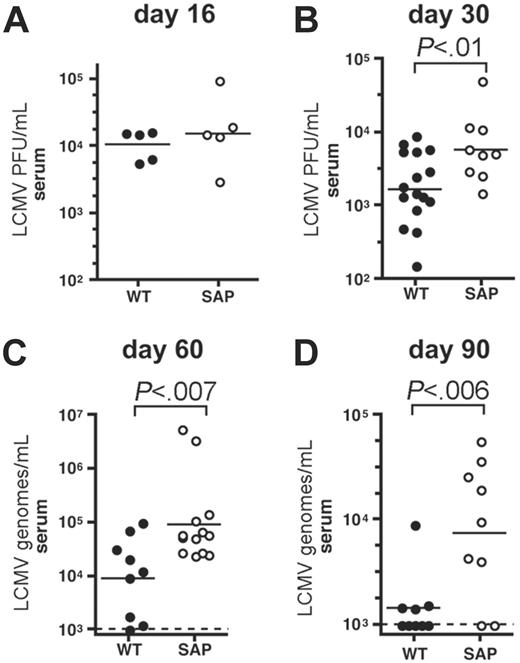
Prolonged viremia in chronically infected SAP– mice. (A) Viremia in wild-type (WT) and SAP– mice was measured by LCMV plaque assay at day 16 after LCMVcl13 infection. Viral loads were similar in wild-type and SAP– mice (P > .05). (B) Viremia in wild-type and SAP– mice was measured by LCMV plaque assay at day 30 after LCMVcl13 infection. Viral loads were 3-fold higher in SAP– mice than in WT mice (P < .01). (C) Viremia was determined 60 days after infection by QPCR for LCMV genomes per milliliter. Viremia in SAP– mice was 10-fold higher than in WT mice (P < .007). Limit of detection was 103 copies per milliliter. (D) Viremia was determined 90 days after infection by QPCR for LCMV genomes per milliliter. Viremia in SAP– mice was 6-fold higher than in WT mice (P < .006). Composite data from 3 independent experiments are shown.
Prolonged viremia in chronically infected SAP– mice. (A) Viremia in wild-type (WT) and SAP– mice was measured by LCMV plaque assay at day 16 after LCMVcl13 infection. Viral loads were similar in wild-type and SAP– mice (P > .05). (B) Viremia in wild-type and SAP– mice was measured by LCMV plaque assay at day 30 after LCMVcl13 infection. Viral loads were 3-fold higher in SAP– mice than in WT mice (P < .01). (C) Viremia was determined 60 days after infection by QPCR for LCMV genomes per milliliter. Viremia in SAP– mice was 10-fold higher than in WT mice (P < .007). Limit of detection was 103 copies per milliliter. (D) Viremia was determined 90 days after infection by QPCR for LCMV genomes per milliliter. Viremia in SAP– mice was 6-fold higher than in WT mice (P < .006). Composite data from 3 independent experiments are shown.
LCMVcl13 viral loads were tracked in SAP– and wild-type mice. No significant difference in viral loads was observed in SAP– mice 2 weeks after infection (P > .05) (Figure 2A), when viremia is peaking.50,51 By day 30, 5- to 10-fold reductions in serum LCMV PFU per milliliter were observed in most wild-type mice (1.8 × 103 ± 0.45 × 103) (Figure 2B). However, SAP– mice had minimal evidence of viral control at day 30 after infection (P < .01), with near peak levels of LCMV viremia (6.1 × 103 ± 1.8 × 103 PFU/mL) (Figure 2B). Higher viral loads in liver and kidney were also found in SAP– mice at day 30 after infection (data not shown). Wild-type mice control viremia to undetectable PFU/mL between day 40 to 80 (though virus is still detectable by QPCR; Figure 2).51,52 Divergence in viremia between wild-type and SAP– mice became more pronounced at 2 months (P < .007) (Figure 2C) and 3 months after infection (P < .03) (Figure 2D), with approximately 10 times higher viremia present in SAP–-deficient mice. At these later time points viral loads were measured by quantitative real-time PCR (QPCR) of LCMV RNA genomes for substantially greater sensitivity (M.M.M. and S.C., manuscript in preparation). SAP– mice did exhibit some progress lowering viral replication, but containment of viremia was incomplete and was not accompanied by an improvement in health in SAP– mice, in stark contrast with wild-type mice.
Impaired germinal center formation and absence of long-lived plasma cells in chronically infected SAP– mice. B-cell responses in wild-type and SAP– mice chronically infected with LCMVcl13 were measured at day 30 after infection (A-B,E-H). (A) Germinal center B cells in spleen were quantified by cytometry (Fashi PNAhi IgD– B220+). Germinal center responses were 30-fold larger in WT mice (P < .001) (WT, n = 13; SAP–, n = 5). (B) Gated B cells (B220+) are shown. All Fashi PNAhi germinal center B cells were IgD– (not shown). Background staining was 0.2% in an uninfected B6 mouse. (C) For comparison, germinal center B cells were quantified at day 30 after acute infection with LCMVarm. WT responses were 20-fold higher than those of SAP– mice (P < .001). (D) Gated B220+ B cells are shown on day 30 after LCMVarm infection. All Fashi PNAhi germinal center B cells were IgD– (not shown). (E) IgG+ virus-specific plasma cells and plasmablasts (antibody secreting cells [ASC]) in the spleen were quantified by ELISPOT on day 30 after LCMVcl13 infection. WT responses were 9-fold higher than those of SAP– mice (P < .01). (F) Long-lived plasma cells in the bone marrow. Anti-LCMV IgG+ plasma cells were quantified from the femurs using ELISPOT. Nine-fold more anti-LCMV plasma cells were present in WT bone marrow than in SAP– bone marrow (P < .001). (G) Total IgG+ ASCs in spleen were 9-fold more numerous in WT than in SAP– mice (P < .01). (H) Total IgG+ long-lived plasma cells in bone marrow were 8 times more prevalent in WT bone marrow than in SAP– bone marrow (P < .001). **P < .01; ***P < .001.
Impaired germinal center formation and absence of long-lived plasma cells in chronically infected SAP– mice. B-cell responses in wild-type and SAP– mice chronically infected with LCMVcl13 were measured at day 30 after infection (A-B,E-H). (A) Germinal center B cells in spleen were quantified by cytometry (Fashi PNAhi IgD– B220+). Germinal center responses were 30-fold larger in WT mice (P < .001) (WT, n = 13; SAP–, n = 5). (B) Gated B cells (B220+) are shown. All Fashi PNAhi germinal center B cells were IgD– (not shown). Background staining was 0.2% in an uninfected B6 mouse. (C) For comparison, germinal center B cells were quantified at day 30 after acute infection with LCMVarm. WT responses were 20-fold higher than those of SAP– mice (P < .001). (D) Gated B220+ B cells are shown on day 30 after LCMVarm infection. All Fashi PNAhi germinal center B cells were IgD– (not shown). (E) IgG+ virus-specific plasma cells and plasmablasts (antibody secreting cells [ASC]) in the spleen were quantified by ELISPOT on day 30 after LCMVcl13 infection. WT responses were 9-fold higher than those of SAP– mice (P < .01). (F) Long-lived plasma cells in the bone marrow. Anti-LCMV IgG+ plasma cells were quantified from the femurs using ELISPOT. Nine-fold more anti-LCMV plasma cells were present in WT bone marrow than in SAP– bone marrow (P < .001). (G) Total IgG+ ASCs in spleen were 9-fold more numerous in WT than in SAP– mice (P < .01). (H) Total IgG+ long-lived plasma cells in bone marrow were 8 times more prevalent in WT bone marrow than in SAP– bone marrow (P < .001). **P < .01; ***P < .001.
Drastically reduced germinal centers and plasma cells in chronically infected SAP– mice
B-cell responses are blocked at the germinal center stage after acute viral infection or protein immunization of SAP– mice12,53,54 (Figure 3C-D). It was therefore of interest to determine the extent of the B-cell response defect in the context of chronic antigen stimulation and high antigen loads. Would chronic antigen stimulation constantly maintain an extrafollicular B-cell response (responsible for the bulk of the early T-cell–dependent short-lived plasma cell response seen during an acute infection,55,56 which is predominantly SAP independent)12 or even force a productive germinal center response in the absence of SAP?
Germinal center B-cell responses were measured in LCMVcl13-infected mice by flow cytometry. Quantitation revealed that SAP– mice had a meager 30 000 germinal center B cells per spleen, 30 times fewer than present in wild-type mice (920 000; P < .001) (Figure 3A-B). In addition, the strong early extrafollicular B-cell response was not maintained in chronically infected SAP– mice, because there were minimal virus-specific plasma cells in the spleen at day 30 after infection (760 ± 570), 9 times fewer than in wild-type mice (P < .01) (Figure 3E). SAP– mice also failed to accumulate virus-specific long-lived plasma cells in the bone marrow (P < .001) (Figure 3F), demonstrating an inability of SAP– mice to generate key components of long-term humoral immunity even in the context of an ongoing infection. Intriguingly, these defects were also reflected in the numbers of total plasma cells in spleen and bone marrow (P < .01 and P < .001, respectively) (Figure 3G-H).
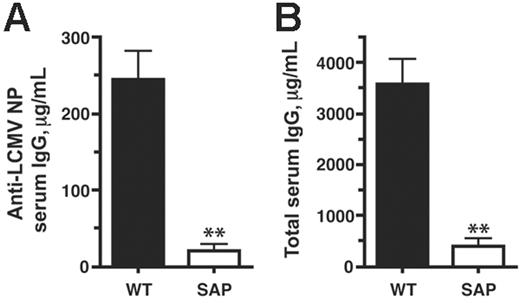
We then examined the effects of these B-cell response defects on levels of serum antibody. Anti-LCMV nucleoprotein (NP) IgG levels in SAP– mice were greatly reduced in comparison with wild-type levels (21 μg/mL versus 244 μg/mL; P < .007) (Figure 4A). Moreover, this defect was also reflected in significantly reduced total serum IgG levels in SAP– mice at day 30 after infection (490 μg/mL versus 3600 μg/mL; P < .003) (Figure 4B). This progressive hypogammaglobulinemia is consistent with the phenotype of XLP humans.
SAP– T-cell function in chronic infection
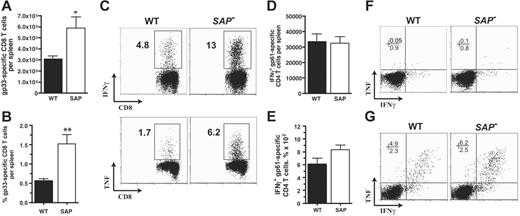
CD8 T cells, CD4 T cells, and B cells are all required to control a chronic LCMV infection.48,57-64 In SAP– mice a striking 90% increased CD8 T-cell response to LCMVcl13 was observed at day 8 after infection (5.9 × 105 versus 3.1 × 105; P < .03) (Figure 5A), consistent with the heightened CD8 T-cell responses observed in acute viral infection (Czar et al10 ;Wuetal11 ; and S.C., unpublished data) and γ-herpesvirus 68 infection.13,22 The percentage of gp33-specific effector CD8 T cells in spleen was 3-fold increased in SAP– mice (Figure 5B-C). Production of IFNγ and TNF (mean fluorescence intensity) per antigen-specific CD8 T cell was comparable between LCMVcl13-infected SAP– and wild-type mice (Figure 5C), while overall there was an increase in IFNγ and TNF production in SAP– mice due to the large increase in the number and frequency of antigen-specific CD8 T cells (Figure 5C). In comparison with acute LCMVarm infection, LCMVcl13 chronic infection of wild-type or SAP– mice resulted in greatly reduced cytokine production by CD4 T cells ex vivo (Figure 5F-G). These observations are attributed to early exhaustion of T cells in a chronic LCMV infection.52,65 Comparable CD4 T-cell responses to LCMVcl13 were observed in both SAP– and wild-type B6 mice (Figure 5D-F).
Impaired antibody response during a chronic viral infection in SAP– mice. Serum antibody levels were determined by quantitative ELISA at day 30 of chronic LCMVcl13 infection. (A) Anti-LCMV serum IgG concentration was 12-fold higher in WT than in SAP– mice (P < .007) (WT, n = 13; SAP–, n = 4). Data are representative of 3 independent experiments. (B) Total serum IgG concentration was 9-fold higher in WT than in SAP– mice (P < .003).
Impaired antibody response during a chronic viral infection in SAP– mice. Serum antibody levels were determined by quantitative ELISA at day 30 of chronic LCMVcl13 infection. (A) Anti-LCMV serum IgG concentration was 12-fold higher in WT than in SAP– mice (P < .007) (WT, n = 13; SAP–, n = 4). Data are representative of 3 independent experiments. (B) Total serum IgG concentration was 9-fold higher in WT than in SAP– mice (P < .003).
SAP– effector CD8 and CD4 T-cell responses to chronic viral infection. Antiviral CD8 and CD4 T-cell responses were measured at day 8 after LCMVcl13 infection (A-F). (A) LCMV-specific CD8+ T cells in spleen were quantified at day 8 after infection by gp33-41 Db MHC I tetramer binding using flow cytometry. SAP– LCMV-specific CD8 T cells were 90% more numerous (P < .03) (WT, n = 7; SAP–, n = 8). Data were pooled from 2 independent experiments. (B) Gp33-specific CD8 T cells are also shown as percentage of total splenocytes (P < .003). (C) IFNγ and TNF production profiles of W and SAP– CD8 T cells after 5 hours stimulation with gp33-41. (D) LCMV-specific CD4+ T cells in spleen were quantified by 5 hours of stimulation with the immunodominant gp61-80 I-Ab MHC II peptide and then analyzed by intracellular cytokine staining for IFNγ production. (E) Gp61-specific CD4 T cells as percentage of total splenocytes. (F) Intracellular cytokine staining for TNF and IFNγ after stimulation of day 8 post-LCMVcl13 infection spleen cells with gp61-80 peptide. CD4+ gated cells are shown. (G) For comparison, CD4 T cells at day 8 after acute LCMVarm infection. Intracellular cytokine staining for TNF and IFNγ after stimulation with gp61-80 peptide. CD4+ gated cells are shown. *P < .05. **P < .01. Data are representative of 4 independent experiments.
SAP– effector CD8 and CD4 T-cell responses to chronic viral infection. Antiviral CD8 and CD4 T-cell responses were measured at day 8 after LCMVcl13 infection (A-F). (A) LCMV-specific CD8+ T cells in spleen were quantified at day 8 after infection by gp33-41 Db MHC I tetramer binding using flow cytometry. SAP– LCMV-specific CD8 T cells were 90% more numerous (P < .03) (WT, n = 7; SAP–, n = 8). Data were pooled from 2 independent experiments. (B) Gp33-specific CD8 T cells are also shown as percentage of total splenocytes (P < .003). (C) IFNγ and TNF production profiles of W and SAP– CD8 T cells after 5 hours stimulation with gp33-41. (D) LCMV-specific CD4+ T cells in spleen were quantified by 5 hours of stimulation with the immunodominant gp61-80 I-Ab MHC II peptide and then analyzed by intracellular cytokine staining for IFNγ production. (E) Gp61-specific CD4 T cells as percentage of total splenocytes. (F) Intracellular cytokine staining for TNF and IFNγ after stimulation of day 8 post-LCMVcl13 infection spleen cells with gp61-80 peptide. CD4+ gated cells are shown. (G) For comparison, CD4 T cells at day 8 after acute LCMVarm infection. Intracellular cytokine staining for TNF and IFNγ after stimulation with gp61-80 peptide. CD4+ gated cells are shown. *P < .05. **P < .01. Data are representative of 4 independent experiments.
Immunopathology
Clinical illness after LCMVcl13 infection is primarily caused by immunopathology of the host immune response to the virus,49,66 as the virus itself causes minimal pathology because it is poorly cytopathic and readily establishes high-titer “carrier” infections in neonatal mice (natural carriers) or immunodeficient mice (eg, RAG–/–).49,67 Because SAP– mice exhibited greater clinical illness and heightened CD8 T-cell responses after infection, we examined pathology in LCMVcl13-infected SAP– mice in detail. In addition to more severe weight loss (Figure 1), we observed extensive damage to lymphoid tissues in SAP– mice (Figure 6). SAP– spleens were visibly smaller and acellular compared with wild-type spleens. Spleen cell counts revealed that infected SAP– spleens were 25% the size of wild-type spleens (P < .001) (Figure 6B). Pathology in spleen tissue was examined histologically. Extensive destruction of splenic architecture was observed in SAP– mice, with generalized disruption of white pulp and leakage of red blood cells (Figure 6A). Bone marrow immunopathology was also present, as total numbers of femur bone marrow cells were reduced 30% to 50% in SAP– versus wild-type mice infected with LCMVcl13 (data not shown).
T-cell responses 1 month after infection
Antiviral T-cell responses were assessed at 30 days after infection in SAP– and wild-type mice (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article), a time point when LCMVcl13 is replicating at high levels in many tissues and T-cell functions are known to be reduced in wild-type mice.51,52,68 This loss of T-cell cytokine production and effector functions is termed “exhaustion,” and it becomes progressively worse in the presence of high viral load and recovers when viral load diminishes.51,52,68,69 The percentage of LCMV-specific CD8 T cells was similar between wild-type and SAP– mice at day 30 after infection (Figure S1A). Comparable results were seen for the antiviral CD4 T-cell response (Figure S1C). Because SAP and SLAM have a role in the regulation of cytokine production,10,11,14,15 it was of interest to determine whether SAP– T cells undergo the loss of cytokine production that is a hallmark of high viral load chronic infections. Although SAP– CD8 T cells still exhibited some responsiveness to antigenic stimulation, IFNγ production per cell after LCMV gp33 peptide stimulation of CD8 T cells was reduced in both wild-type and SAP– mice (Figure S1B). Similar results were observed for ex vivo–stimulated LCMV-specific CD4 T cells (Figure S1D).
Immunopathology. (A) Spleen histology day 15 after infection with LCMVcl13. Sections were fixed in formalin and then counterstained with hematoxylin and eosin. A representative white pulp region is shown for both WT and SAP– mice. Aggressive destruction of the lymphoid architecture is visible in SAP– mice, with extensive leakage of red blood cells into the white pulp. (B) Spleen cellularity was significantly reduced in SAP– mice 2 weeks after LCMVcl13 (P < .001). (Composite of 2 experiments [day 13 and 14]. WT, n = 6 [2 and 4]; SAP–, n = 5 [2 and 3].). Histologic data are representative of 3 independent experiments.
Immunopathology. (A) Spleen histology day 15 after infection with LCMVcl13. Sections were fixed in formalin and then counterstained with hematoxylin and eosin. A representative white pulp region is shown for both WT and SAP– mice. Aggressive destruction of the lymphoid architecture is visible in SAP– mice, with extensive leakage of red blood cells into the white pulp. (B) Spleen cellularity was significantly reduced in SAP– mice 2 weeks after LCMVcl13 (P < .001). (Composite of 2 experiments [day 13 and 14]. WT, n = 6 [2 and 4]; SAP–, n = 5 [2 and 3].). Histologic data are representative of 3 independent experiments.
CD8 T-cell–dependent immunopathology
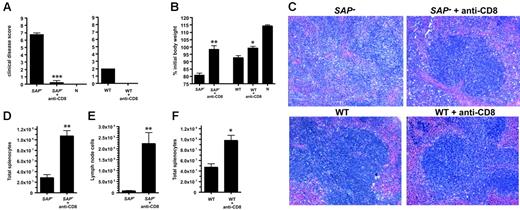
Severe splenic hypoplasia and tissue architecture disruption in SAP– mice was indicative of greater immunopathology in the absence of SAP function. Excessive CD8 T-cell activation early in infection was a likely cause of the exacerbated LCMVcl13 disease severity, because CD8 T cells play major roles both in clearance of LCMV and organ damage from cytotoxic T-cell activity.49,57,66-68,70-72 Therefore, we directly tested the role of CD8 T cells in the aggressive immunopathology observed in SAP– mice by depleting CD8 T cells prior to infection. CD8 T cells were completely depleted from all animals (data not shown). Immunopathology in intact or CD8 T-cell–depleted SAP– mice was scored by 5 independent measures: clinical disease score, weight loss after infection, spleen histology, spleen size, and axillary lymph node size. SAP– mice exhibited severe clinical illness symptoms by 1 week after infection (ruffled fur, lack of mobility, hunched back), while SAP– mice without CD8 T cells exhibited no overt disease (P < .01) (Figure 7A). SAP– mice rapidly lost weight, but weight was stable in CD8-depleted SAP– mice (P < .01) (Figure 7B). The more moderate disease symptoms in wild-type mice were also improved by CD8 T-cell depletion, as expected (Figure 7A-B,F).72 (Uninfected mice continued to gain weight, but high viral loads in LCMVcl13-infected SAP– and wild-type mice stunt growth irrespective of the CD8 T-cell response.) Immunopathology was examined histologically in spleen sections from intact or CD8-depleted SAP– mice. Immunopathology and disruption of splenic architecture (white pulp destruction, red blood cell infiltration) was severe in intact SAP– mice but was greatly reduced in CD8-depleted SAP– mice (Figure 7C). Examination of killed mice revealed striking splenic hypoplasia in SAP– mice but not CD8-depleted mice (P < .01) (Figure 7D). SAP– mice peripheral lymph nodes were destroyed almost completely, with few remaining live lymphocytes, while CD8-depleted SAP– mice had easily identifiable lymph nodes with much greater cellularity (P < .01) (Figure 7E).
SAP– immunopathology is CD8 T-cell mediated.SAP– mice and SAP– mice depleted of CD8 T cells (SAP– + anti-CD8) were infected with 4 × 106 PFU LCMVcl13 and examined for immunopathology. (A) Clinical illness. Mice were examined visually and assigned a composite score based on signs of illness (ruffled fur, mobility, hunched back; maximum possible score, 9; normal health, 0). CD8-depleted SAP– mice were significantly healthier than normal SAP– mice (P < .001) (left panel); n indicates uninfected, naive control mouse. Clinical disease was also ameliorated in WT mice depleted of CD8 T cells, as expected (right panel). SAP–,n = 4; SAP– + anti-CD8; n = 4; WT, n = 4; WT + anti-CD8, n = 4; naives, n = 2. (B) Body weight 1 week after infection. Weight loss was significantly worse in normal SAP– mice than in CD8-depleted SAP– mice (P < .01). (C) Spleen histology day 8 after infection. Sections were fixed in formalin and then counterstained with hematoxylin and eosin. A representative white pulp region is shown for SAP– and SAP– + anti-CD8 tissue sections (top panels). Extensive disruption of the white pulp and splenic architecture is present in SAP– mice, in contrast with SAP– mice treated with anti-CD8 antibody. Bottom panels show sections from treated and untreated WT mice infected with LCMVcl13. (D) Spleen cell counts day 8 after infection. Spleens from intact SAP– mice were significantly smaller and more acellular than spleens from CD8-depleted SAP– mice (P < .01). (E) Lymph node (axillary) cell counts day 8 after infection. (F) Wild-type spleen cell counts day 8 after infection. All data are representative of 3 independent experiments. *P < .05. **P < .01. ***P < .001.
SAP– immunopathology is CD8 T-cell mediated.SAP– mice and SAP– mice depleted of CD8 T cells (SAP– + anti-CD8) were infected with 4 × 106 PFU LCMVcl13 and examined for immunopathology. (A) Clinical illness. Mice were examined visually and assigned a composite score based on signs of illness (ruffled fur, mobility, hunched back; maximum possible score, 9; normal health, 0). CD8-depleted SAP– mice were significantly healthier than normal SAP– mice (P < .001) (left panel); n indicates uninfected, naive control mouse. Clinical disease was also ameliorated in WT mice depleted of CD8 T cells, as expected (right panel). SAP–,n = 4; SAP– + anti-CD8; n = 4; WT, n = 4; WT + anti-CD8, n = 4; naives, n = 2. (B) Body weight 1 week after infection. Weight loss was significantly worse in normal SAP– mice than in CD8-depleted SAP– mice (P < .01). (C) Spleen histology day 8 after infection. Sections were fixed in formalin and then counterstained with hematoxylin and eosin. A representative white pulp region is shown for SAP– and SAP– + anti-CD8 tissue sections (top panels). Extensive disruption of the white pulp and splenic architecture is present in SAP– mice, in contrast with SAP– mice treated with anti-CD8 antibody. Bottom panels show sections from treated and untreated WT mice infected with LCMVcl13. (D) Spleen cell counts day 8 after infection. Spleens from intact SAP– mice were significantly smaller and more acellular than spleens from CD8-depleted SAP– mice (P < .01). (E) Lymph node (axillary) cell counts day 8 after infection. (F) Wild-type spleen cell counts day 8 after infection. All data are representative of 3 independent experiments. *P < .05. **P < .01. ***P < .001.
Discussion
Human SAP deficiency and lethal Epstein-Barr virus (EBV) infection are strongly linked. To understand that linkage and the importance of SAP in lymphocyte functions, it is necessary to understand SAP functions that are broadly involved in adaptive immune responses versus SAP immunobiology specifically related to EBV pathogenesis, because XLP patients sometimes also have severe difficulties with other viral and bacterial infections and chronic ailments related to infections.28-31 Here we have addressed these issues by examining SAP– mice immune responses and pathogenesis during a chronic LCMV infection as a model of human XLP clinical disease.
Multifaceted B-cell response defects
We previously showed that SAP is a key regulator of humoral immune responses during an acute immune response.12 All IgG antibody production after LCMV infection is T-cell dependent (Whitmire et al73 ; M.M.M. and S.C., manuscript submitted), and we previously determined that the humoral immunity defect in SAP– mice was due to a requirement for SAP in CD4 T cells to generate germinal center B cells,12 and germinal centers are the source of memory B cells and the likely source of long-lived plasma cells.56 In addition, the long-term humoral immunity defect has now been confirmed in humans, as XLP patients have a striking 10-fold reduction in memory B cells, indicating that they have a major germinal center block comparable to the phenotype we reported in SAP– mice.7,26,27
Here we showed SAP– mice exhibit a severe block in germinal center formation and an absence of long-lived plasma cells during a chronic viral infection (Figure 3), demonstrating that the requirement for SAP cannot be overcome by the presence of constant antigenic stimulation during a chronic infection. Indeed, by 1 month after infection there was little evidence of any ongoing anti-LCMV humoral immune response in the SAP– mice, and serum antibody levels were sharply reduced (Figure 3, 4). This also indicates that an extrafollicular B-cell response is short-lived and cannot be extended to maintain an antiviral IgG response, even in the absence of germinal center formation (which possibly normally drains the relevant B- and T-cell resources away from the extrafollicular response).
We speculate that the absence of germinal centers may be a particularly problematic “double hit” in humans with SAP deficiency, because (1) the absence of an affinity matured antibody response may lead to ineffective control of EBV (though CD4 T cells, CD8 T cells, and NK cells are expected to be more critical) and other infections, and (2) the elimination of a normal germinal center microenvironment and memory B cells removes natural EBV latency habitats, as initially postulated by Thorley-Lawson.74 The absence of germinal centers may therefore drastically skew the pathology of EBV disease by forcing the virus to maintain lytic replication in other available B-cell compartments instead of switching into latency via germinal center B cells, resulting in altered viral tropism in SAP– cells and greater disease.
It is also important that a primary clinical therapy for XLP patients is ongoing treatment with intravenous human immunoglobulin (IVIG).30,33-35,75 Our B-cell response results in SAP– mice give a parsimonious hypothesis for the value of IVIG in SAP-deficient humans: In the absence of SAP, germinal center formation is blocked and the ability to generate affinity matured antibodies, establish long-term antibody production (via long-lived plasma cells), and produce memory B cells are all severely abrogated. Therefore, IVIG provides the missing antibodies, and the relatively intact SAP– T-cell compartment is able to control many viral infections in combination with the passively provided antibodies. The ability of SAP– mice to make acute T-dependent antibody responses suggests that SAP-deficient humans should be able to control many acute infections requiring antibodies for pathogen control and clearance unless an affinity matured antibody response is required. IVIG perhaps provides an additional level of protection by serving as a source of affinity matured antibodies to many pathogens (including EBV) and mimicking long-term serum antibody maintenance by repeated IVIG injections. These speculations are consistent with observations in XLP patients.30,31,36,76
T-cell hyperproliferation, viral control, and immunopathology
Greater CD8 and CD4 T-cell expansion after infection has been a signature of SAP– mouse immune responses to acute viral infection.10-12 Here we showed that SAP– mice also make a 2-fold to 3-fold heightened effector CD8 T-cell response to a chronic LCMVcl13 infection (Figure 5). Importantly, in a chronic LCMV infection, SAP– mice exhibited severe immunopathology (Figure 6), and that immunopathology is caused by the increased number of SAP– effector CD8 T cells, because the immunopathology and clinical disease can be blocked by depletion of the SAP– CD8 T cells (Figure 7). This is the first direct demonstration of SAP– CD8 T-cell–mediated immunopathology. Human XLP disease is associated with CD8 T-cell hyperproliferation and multiorgan tissue infiltration,29,30,77 and our results provide a direct causal link for those observations.
Increased CD8 T-cell responses are relatively benign in the context of an acute infection, because total and rapid clearance of the viral antigens is desired. Indeed, most infectious virions are cleared by day 6 of an acute infection with LCMV Armstrong strain in wild-type and SAP– mice (S.C., data not shown). That is before the bulk of the excessive SAP– T-cell expansion occurs, leaving those excess CD8 T cells without sources of antigenic stimulation or targets to kill. However, excessive effector CD8 T-cell expansion is of grave consequence in an extended viral infection—such as EBV in SAP-deficient humans or LCMVcl13 in SAP– mice— because those extra activated T cells can rapidly cause severe immunopathology and even death. SAP– LCMV-specific effector CD8 T cells produce TNF and IFNγ (Figure 5) and are efficient cytotoxic T cells.10 TNF, perforin, IFNγ, and FasL have all been implicated in LCMV immunopathology and clinical illness in a variety of experimental models,49,66,78-82 and a more detailed examination of the contributions of individual CD8 T-cell effector functions to the immunopathology observed in SAP– mice warrants further examination.
It can be considered counterintuitive that the more active SAP– CD8 T-cell response did not result in better control of LCMVcl13 replication. It is important to understand, however, that if the virus replicates fast enough to quickly reach high viral loads in many tissues before the presence of a strong CD8 T-cell response and if the virus is itself noncytopathic, cytotoxic T-cell functions quickly become detrimental to the host. Indeed, as early as day 7 after infection, the T-cell response to LCMVcl13 begins to diminish in wild-type mice due to a self-preservation mechanism in the host to stop immunopathology and prevent death.65,68 In contrast, excessive numbers of antigen-specific SAP– CD8 T cells resulted in increased cytokine production and killing of infected cells and severe immunopathology within the first 2 weeks of infection (Figures 1 and 6), which dramatically compromised lymphoid organs (spleen, lymph nodes, and bone marrow) and general health such that SAP-deficient animals were no longer able to control the chronic viral infection over time. In addition, both B and CD4 T-cell functions are required to control a chronic LCMV infection62,63,67 (NK cells are not major contributors to the control of LCMV83-85 ), and therefore the inability of SAP– mice to generate and sustain high levels of antiviral antibodies probably also contributed to the inability of SAP– mice to blunt and control LCMVcl13 infection.
Two groups have reported studies on SAP– mice infected with γ-herpesvirus 68 (MHV-68), the closest known murine relative to EBV. In a crisp set of experiments, Huber's group22 reported that SAP– mice have higher CD8 T-cell responses and better control of chronic MHV-68 infection. In contrast, with a higher-dose infection, Romeo's group13 observed higher levels of MHV-68 replication in SAP– mice along with more activated CD8 T cells, with pathology similar to virus-associated hemophagocytic syndrome (VAHS). Both of those sets of observations are consistent with our findings, and they are consistent with our hypothesis that viral control versus immunopathology by SAP– CD8 T cells is likely strongly influenced by the tropism of the viral infection and the magnitude of the viral load irrespective of whether the infection is a herpesvirus or a nonherpesvirus. A high-dose infection with MHV-68 gave similar results to LCMVcl13 infection (increased immunopathology and insufficient viral control).13 The lower-dose MHV-68 infection resulted in better control of MHV-68,22 we hypothesize, because under those conditions the race between CD8 T cells and the virus changed the balance to favor the larger CD8 T-cell response capable of limiting the early infection before the infection expanded to reach an overwhelming viral load where the CD8 T-cell response against the infected cells would result in immunopathology.
In conclusion, our findings in a nonherpesvirus chronic viral infection mouse model recapitulate 2 key features of human XLP—namely, progressive hypogammaglobulinemia and severe CD8 T-cell–mediated immunopathology—showing the extensive effects of SAP dysregulation on lymphocyte functions involved in control of viral infection. From these findings and other results in the literature we can posit that a combination of factors is likely to be responsible for the phenotypes observed in XLP patients. The progressive hypogammaglobulinemia in SAP-deficient humans is likely due to the block in germinal center formation and long-lived plasma cell production observed in SAP– mice (Figures 3, 4). The ability to control common acute viral and bacterial infections may be due to a relatively intact CD8 T-cell compartment, CD4 T-cell compartment, and short-term T-dependent antibody responses. And the special difficulty of SAP-deficient patients in dealing with EBV and certain other infections may stem from a combination of the severe NK function defects in the absence of SAP,18-20 hyperproliferative CD8 T-cell responses resulting in starkly exacerbated immunopathology in the presence of persisting virus (Figures 1 and 6, 7), absence of functional germinal centers (altering EBV tropism and pathogenesis), absence of affinity matured and persisting antibody response, and the fact that EBV is so prevalent in the human population (more than 90% infection rate).
Prepublished online as Blood First Edition Paper, June 20, 2006; DOI 10.1182/blood-2006-04-018929.
Funded by a Pew Scholar Award and a Cancer Research Institute Investigator Award (S.C.).
S.C. designed and performed experiments, analyzed data, and wrote the paper; M.M.M. performed experiments; R.D.A. performed preliminary experiments; and E.J.W. and R.A. designed experiments and analyzed data.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Pamela Schwartzberg, Dr Hilde Cheroutre, Dr Charlie Surh, Dr Stephen Schoenberger, and Dr Michael Buchmeier for mice, MHC I tetramer, and antibodies. We thank Nathalie Droin, Sara McBride, Dr Luca Guidotti, and Dr Matteo Iannacone for assistance with the QPCR assay; Dr Juan de la Torre for plasmid; Tom Wolfe for assistance with plaque assays; and Dr Loui Madakamutil for assistance with tetramers. We thank Dr Stephen Schoenberger and Dr Matthias von Herrath for sharing equipment. And we thank Chuck Prickett and Fernando Vasquez for excellent assistance with animal care.

![Figure 3. Impaired germinal center formation and absence of long-lived plasma cells in chronically infected SAP– mice. B-cell responses in wild-type and SAP– mice chronically infected with LCMVcl13 were measured at day 30 after infection (A-B,E-H). (A) Germinal center B cells in spleen were quantified by cytometry (Fashi PNAhi IgD– B220+). Germinal center responses were 30-fold larger in WT mice (P < .001) (WT, n = 13; SAP–, n = 5). (B) Gated B cells (B220+) are shown. All Fashi PNAhi germinal center B cells were IgD– (not shown). Background staining was 0.2% in an uninfected B6 mouse. (C) For comparison, germinal center B cells were quantified at day 30 after acute infection with LCMVarm. WT responses were 20-fold higher than those of SAP– mice (P < .001). (D) Gated B220+ B cells are shown on day 30 after LCMVarm infection. All Fashi PNAhi germinal center B cells were IgD– (not shown). (E) IgG+ virus-specific plasma cells and plasmablasts (antibody secreting cells [ASC]) in the spleen were quantified by ELISPOT on day 30 after LCMVcl13 infection. WT responses were 9-fold higher than those of SAP– mice (P < .01). (F) Long-lived plasma cells in the bone marrow. Anti-LCMV IgG+ plasma cells were quantified from the femurs using ELISPOT. Nine-fold more anti-LCMV plasma cells were present in WT bone marrow than in SAP– bone marrow (P < .001). (G) Total IgG+ ASCs in spleen were 9-fold more numerous in WT than in SAP– mice (P < .01). (H) Total IgG+ long-lived plasma cells in bone marrow were 8 times more prevalent in WT bone marrow than in SAP– bone marrow (P < .001). **P < .01; ***P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/9/10.1182_blood-2006-04-018929/4/m_zh80210602880003.jpeg?Expires=1767771400&Signature=wrkTjPr2YU4MM6I5uJO0ht5oQvzTkAhzeIXrSOTYRvVlwVF5reLU0CUzjKkmiW6MQs0ASN83VucEkeP25WDowP~zbp8b8fdelrRnBo-wwcc~7UGLmNQKfSOVKFLtRweX1plIbJKEIAqg6bcgSV8OudOBbrhef6RcSjx-RifEBwfu1oREhtzqomj9hYQnrSNSZUh-oUrSd6XPgvz8orVqObrsgWt0kx6BynXu0n0qQA8xkpXaGFIFoCOQ2ubrDb2Asu44vanWJ7Squ1IfGwvYKfN7vFHtLymliQ9CAM~X5fKUtjaN9Q6liv87BTKytMdkyAJN4KkyAHh5aw4wMegiMw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Immunopathology. (A) Spleen histology day 15 after infection with LCMVcl13. Sections were fixed in formalin and then counterstained with hematoxylin and eosin. A representative white pulp region is shown for both WT and SAP– mice. Aggressive destruction of the lymphoid architecture is visible in SAP– mice, with extensive leakage of red blood cells into the white pulp. (B) Spleen cellularity was significantly reduced in SAP– mice 2 weeks after LCMVcl13 (P < .001). (Composite of 2 experiments [day 13 and 14]. WT, n = 6 [2 and 4]; SAP–, n = 5 [2 and 3].). Histologic data are representative of 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/108/9/10.1182_blood-2006-04-018929/4/m_zh80210602880006.jpeg?Expires=1767771400&Signature=AOI0ZpKt8CP9K8htOaXn6wHY8Qlv9Cp30fRsYFp1HKYCijDFcrAAVFRWzy1FnveG4Zcr6KtoXcw~2BiGVbZWrtNmQYVDwWEVVzHHWJ66R4Q2amTIG39A5ZztyXrTOvZchH4-QHpOvABi5y3G2f3iV1G~BNcAN7GqCZFQRzXaC~1nuvzTBH6c3UOvAfvcufQqExwCeWw74ATavS4LD-kxAw~Uf4U5OE1NZIh67-7qyYCoDdUXeMfwsaIA5gZUX6GUfu6NSVK7dKD24ImfqQ1Ph78QkA0EB4YqpOsF-h0JSVreWDGUUH-RR45VeaFrfMWfwiPnMDsxxKoQ~aN9N~Oudw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)