Abstract
B-cell chronic lymphocytic leukemia (B-CLL) progression is determined by malignant cell extravasation and lymphoid tissue infiltration. We have studied the role and regulation of matrix metalloproteinase-9 (MMP-9) in B-CLL cell migration and invasion. Adhesion of B-CLL cells to the fibronectin fragment FN-H89, VCAM-1, or TNF-α–activated human umbilical vein endothelial cells (HUVECs) up-regulated MMP-9 production, measured by gelatin zymography. This effect was mediated by α4β1 integrin and required PI3-K/Akt signaling. The chemokine CXCL12 also up-regulated MMP-9, independently of α4β1 and involving ERK1/2 but not Akt activity. Accordingly, α4β1 engagement activated the PI3-K/Akt/NF-κB pathway, while CXCL12/CXCR4 interaction activated ERK1/2/c-Fos signaling. Anti–MMP-9 antibodies, the MMP-9 inhibitor TIMP-1, or transfection with 3 different MMP-9 siRNAs significantly blocked migration through Matrigel or HUVECs. Cell-associated MMP-9 was mainly at the membrane and contained the proactive and mature forms. Moreover, B-CLL cells formed podosomes upon adhesion to FN-H89, VCAM-1, or fibronectin; MMP-9 localized to podosomes in a PI3-K–dependent manner and degraded a fibronectin/gelatin matrix. Our results are the first to show that MMP-9 is physiologically regulated by α4β1 integrin and CXCL12 and plays a key role in cell invasion and transendothelial migration, thus contributing to B-CLL progression. MMP-9 could therefore constitute a target for treatment of this malignancy.
Introduction
B-cell chronic lymphocytic leukemia (B-CLL) is characterized by the accumulation of monoclonal, slow-dividing CD5+ B lymphocytes in the peripheral blood.1-3 In most cases, these cells progressively infiltrate the bone marrow and secondary lymphoid tissue, resulting in poor prognosis.1-3 Extravasation of B-CLL cells and migration through endothelium are mainly directed by 3 chemokines: CCL21, which is expressed in high endothelial venules (HEVs), and CCL19 and CXCL12, which are produced by stromal cells of lymph nodes and bone marrow, respectively.4,5 The corresponding receptors for these chemokines, CCR7 (for CCL21 and CCL19) and CXCR4 (for CXCL12), are highly expressed in B-CLL with widespread involvement of lymph nodes.5,6
Other molecules such as vascular endothelial growth factor (VEGF) and αLβ2/α4β1 integrins were also recently shown to be involved in B-CLL transendothelial migration in response to chemokines.7 Moreover, high expression of α4β1 (but not αLβ2) correlated with the presence of lymphadenopathy,7 suggesting an important role for this integrin in B-CLL progression.
Transendothelial migration and organ invasion of malignant cells also require proteolytic degradation of the vascular basement membrane and the extracellular matrix of lymphoid tissues. This can be accomplished by matrix metalloproteinases (MMPs),8-10 in particular the gelatinases MMP-2 and MMP-9. MMPs also release matrix-bound growth factors that stimulate malignant cell expansion and angiogenesis.11 Indeed angiogenesis is increased in the bone marrow of B-CLL patients,12-14 and high levels of the angiogenic factors VEGF and basic fibroblast growth factor (bFGF) have been detected in the urine and serum of these patients.12,15,16
Previous studies have shown that early-stage B-CLL cells produce and secrete MMP-9, which can be detected in the serum of these patients and in B-CLL cell culture supernatants.17 It was later demonstrated that B-CLL cells constitutively produce MMP-9 in various molecular forms and that elevated levels of intracellular MMP-9 correlate with advanced stage and poor patient survival.18 Moreover, MMP-9 was highly expressed by B-CLL cells present in the bone marrow (with a diffused pattern) and in lymph nodes, and contributed to B-CLL migration through artificial basement membranes or endothelial cells.18 The presence of other MMPs in B-CLL cells has not been reported.
These previous reports suggest that MMP-9 contributes to B-CLL progression by facilitating malignant cell migration and tissue invasion, and could constitute a target for therapeutic intervention. It is therefore important to understand the mechanisms that regulate MMP-9 production in B-CLL. To address this, we have studied the role of α4β1 integrin and CXCR4, 2 molecules involved in B-CLL cell migration, in regulating MMP-9. We show that engagement of either receptor up-regulates MMP-9, and we have identified the signaling pathways involved in this effect. We also show that MMP-9 localizes to podosomes upon adhesion to α4β1 integrin ligands, and plays a crucial role in B-CLL cell transendothelial migration and invasion through basement membranes.
Materials and methods
Patients, cell purification, and cell lines
Peripheral blood samples were obtained after informed consent from 12 B-CLL patients, diagnosed according to established clinical and laboratory criteria. None of them had received treatment at the time of this study, and 8 patients presented lymphadenopathy. CD5+ B lymphocytes were purified by Ficoll-Hypaque (Nycomed, Oslo, Norway) centrifugation. B lymphocytes from healthy donors were purified from buffy coat cells by Ficoll-Hypaque centrifugation and anti–CD19-conjugated Dynabeads (Dynal Biotech ASA, Oslo, Norway). The Epstein-Barr virus (EBV)–transformed CO43, HUT112, and BRO168 cell lines, established from normal B lymphocytes, have been previously reported.19 Human umbilical vein endothelial cells (HUVECs) were kindly provided by Drs S. Lamas and M. L. Botella (Centro de Investigaciones Biológicas, Madrid, Spain) and cultured in Medium 199 Modified Earle Salts (Gibco, Auckland, New Zealand), containing 15% FCS and 50 μg/mL endothelial cell growth factor. HUVECs were used up to the fourth passage. Cell viability was assessed by flow cytometry using FITC–annexin V (Bender Medsystems, Vienna, Austria) and propidium iodide as described.20
Antibodies, reagents, and proteins
Monoclonal antibodies (mAbs) HP2/1 (anti–α4 integrin subunit), HU5/3 (anti–intercellular adhesion molecule-1, ICAM1), and W6/32 (anti-HLA) were obtained from Dr F. Sánchez-Madrid (Hospital de la Princesa, Madrid, Spain); P1D6 (anti–α5 integrin subunit) has been previously described21 ; anti–vascular cell adhesion molecule-1 (VCAM-1, sc-8304), anti–IκB-α (sc-1643), and anti-Akt1 (sc-5298) were from Santa Cruz Biotechnology (Santa Cruz, CA); TS1/11 (anti–αL integrin subunit) and TS1/18 (anti–β2 integrin subunit) were obtained from Drs A. Corbí and C. Bernabeu (Centro de Investigaciones Biológicas); LEM2/15 (anti–membrane type 1-MMP, MT1-MMP) was obtained from Dr A. G. Arroyo (Centro Nacional de Investigaciones Cardiovasculares, Madrid); anti–MMP-9 and anti–MMP-2 were from NeoMarkers (Labvision, Freemont, CA); and antivinculin was from Sigma-Aldrich (St Louis, MO). Rabbit polyclonal antibodies to phospho-Akt (Ser473), phospho-p44/p42 MAPK, and p44/p42 MAPK were from Cell Signaling (Beverly, MA); rabbit antibodies to MMP-9 were from Chemicon (Temecula, CA). TIMP-1, UO126 (MEK inhibitor), PP2 (Src inhibitor), SB203580 (p38 MAPK inhibitor), Triciribine/API-2 (Akt inhibitor), wortmannin and LY294002 (PI3-K inhibitors), BisI (PKC inhibitor), and pertussis toxin were from Calbiochem (Darmstadt, Germany). The chemokine CXCL12 was from R&D Systems (Minneapolis, MN). Matrigel was from BD Biosciences (Erembodegem, Belgium). FITC-gelatin was from Molecular Probes (Leiden, the Netherlands). Poly-lysine (p-Lys) was from Sigma-Aldrich. Plasma fibronectin was purified as described.21 The fibronectin fragment H89 (FN-H89), containing the CS-1 ligand for α4β1 integrin,21 and VCAM-1 were prepared as reported.22,23 ICAM-1 was obtained from Dr C. Cabañas (Instituto de Farmacología y Toxicología, CSIC, Madrid).
Additional information on methods is available in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article.
Results
Constitutive expression of MMPs in B-CLL cells
To establish the constitutive expression of MMPs in B-CLL, we first analyzed lysates of freshly isolated cells by gelatin zymography. All 12 samples studied contained MMP-9 in the 92-kDa proform (shown in Figures 1A for 5 representative samples). MMP-9 expression was significantly higher in B-CLL cells than in normal peripheral blood B cells, or in EBV-transformed B-cell lines (Figure 1A). The presence of MMP-2 was also analyzed by gelatin zymography (6 samples), flow cytometry after cell permeabilization (3 samples), and reverse-transcription–polymerase chain reaction (RT-PCR; 5 samples) (data not shown). These studies established the absence of MMP-2 in B-CLL cells, confirming previous observations.18
We next examined the presence of MT1-MMP in these cells using flow cytometry. As shown in Figure 1B for patient 2 (and not shown for patients 3 and 4), MT1-MMP was not detected at the B-CLL cell surface and was not induced after treatment with PMA. In the same experiment, PMA induced MT1-MMP expression on control NCI-H929 myeloma cells (Figure 1B). We then analyzed the intracellular presence of this MMP. As shown in Figure 1C, MT1-MMP mRNA was detected in all 5 samples studied, with heterogeneous levels of expression. At the protein level, Western blotting analyses of cell lysates from 4 different samples revealed the reported 64-kDa form of MT1-MMP24 as well as other unidentified bands (Figure 1D). From these results, we concluded that although MT1-MMP is found intracellularly in B-CLL cells, it probably plays a minor role in these cells. Accordingly, subsequent studies were focused on MMP-9.
Constitutive expression of MMPs in B-CLL. (A) Lysates from 2 × 106 fresh B-CLL cells from 5 different patients (P1-P5), peripheral blood B lymphocytes (PB-BL), or EBV-transformed normal B cells (Bro168, CO43, HUT112) were analyzed by gelatin zymography on 10% gels. MMP-9 was identified as the 92-kDa proactive form. Actin was analyzed in identical aliquots from the same lysates by Western blotting and used as control for protein loading. Quantitative values represent the average of 2 different samples for PB-BL and 12 different samples for B-CLL. Error bars indicate standard deviation. *** P < .001. (B) Flow cytometric analysis of MT1-MMP surface expression in B-CLL cells from one patient or NCI-H929 myeloma cells, without (Control) or with PMA (50 ng/mL, 24 hours). (C) RT-PCR analysis of constitutive MT1-MMP mRNA expression in B-CLL cells from patients 1 to 5. BLM melanoma cells were included as positive control. (D) Lysates from 4 different B-CLL samples or BLM cells were analyzed by Western blotting, and MT1-MMP was identified using the LEM2/15 mAb.
Constitutive expression of MMPs in B-CLL. (A) Lysates from 2 × 106 fresh B-CLL cells from 5 different patients (P1-P5), peripheral blood B lymphocytes (PB-BL), or EBV-transformed normal B cells (Bro168, CO43, HUT112) were analyzed by gelatin zymography on 10% gels. MMP-9 was identified as the 92-kDa proactive form. Actin was analyzed in identical aliquots from the same lysates by Western blotting and used as control for protein loading. Quantitative values represent the average of 2 different samples for PB-BL and 12 different samples for B-CLL. Error bars indicate standard deviation. *** P < .001. (B) Flow cytometric analysis of MT1-MMP surface expression in B-CLL cells from one patient or NCI-H929 myeloma cells, without (Control) or with PMA (50 ng/mL, 24 hours). (C) RT-PCR analysis of constitutive MT1-MMP mRNA expression in B-CLL cells from patients 1 to 5. BLM melanoma cells were included as positive control. (D) Lysates from 4 different B-CLL samples or BLM cells were analyzed by Western blotting, and MT1-MMP was identified using the LEM2/15 mAb.
α4β1 integrin–mediated B-CLL cell adhesion up-regulates MMP-9 production
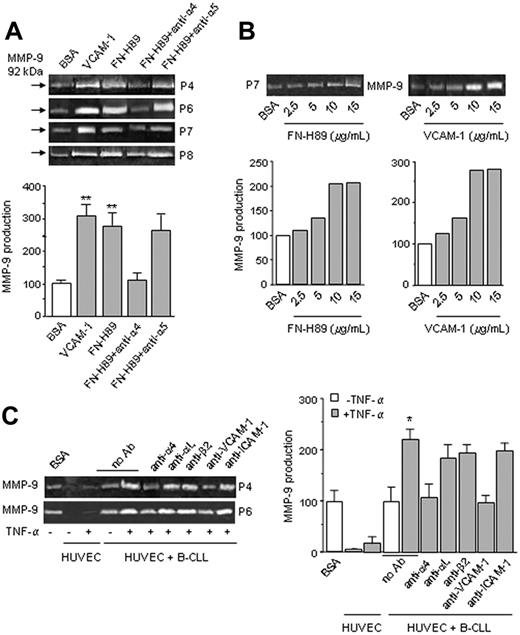
Adhesion to fibronectin mediated by α4β1, α5β1, and αVβ1 integrins or VCAM-1 via α4β1 was previously shown to up-regulate MMP-9 and MMP-2 in T cells.25,26 B-CLL cells encounter fibronectin and VCAM-1 during extravasation and migration within tissues and express α4β1 as a major integrin.27,28 We therefore studied whether MMP-9 was up-regulated in B-CLL cells by interaction with FN-H89 or VCAM-1. Equal number of cells from 6 different patients was incubated on VCAM-1–, FN-H89–, or BSA-coated plates, and after 24 hours the conditioned media were collected and analyzed by gelatin zymography. As shown in Figure 2A for 4 representative samples, and quantitated for all 6 samples studied, MMP-9 was significantly up-regulated by adhesion of B-CLL cells to VCAM-1 or FN-H89, compared with cells cultured on BSA. This effect was completely blocked by anti–α4 integrin subunit mAbs (Figure 2A). It was also blocked by cell preincubation with cycloheximide (not shown), suggesting that MMP-9 up-regulation involved de novo protein synthesis. Enhanced MMP-9 secretion was not due to different viability of cells cultured on FN-H89 or BSA, as confirmed by flow cytometry (Figure S1). Moreover, as shown in Figure 2B for patient 7, up-regulation of MMP-9 by FN-H89 or VCAM-1 was dose dependent, further establishing the specificity of the cell adhesion effect.
α4β1 integrin engagement up-regulates MMP-9 in B-CLL cells. (A) B-CLL cells (2 × 106) from 4 different patients were suspended in RPMI, 0.1% FCS and incubated on wells coated with 0.5% BSA, 10 μg/mL FN-H89, or 10 μg/mL VCAM-1. Some cells were pretreated for 30 minutes with anti-α4 or -α5 mAbs (10 μg/mL) and then incubated in the presence of the Abs. After 24 hours, the conditioned media were concentrated 50 times and analyzed by gelatin zymography. Values for MMP-9 production (arbitrary units) represent the average of all 6 samples studied; basal levels of MMP-9 on BSA were normalized to 100. (B) B-CLL cells from one patient were incubated on wells coated with the indicated concentrations of FN-H89 or VCAM-1, and after 24 hours analyzed as explained. (C) B-CLL cells from 2 patients (5 × 105/100 μL) were incubated on the upper chamber of Transwell filters coated with resting or TNF-α–activated HUVECs, and in the presence or absence of the indicated mAbs. After 24 hours, the conditioned media were concentrated 5 times and analyzed by gelatin zymography. Values for MMP-9 production (arbitrary units) represent the average of the 2 samples studied. Error bars indicate standard deviation. * P < .05; ** P < .01.
α4β1 integrin engagement up-regulates MMP-9 in B-CLL cells. (A) B-CLL cells (2 × 106) from 4 different patients were suspended in RPMI, 0.1% FCS and incubated on wells coated with 0.5% BSA, 10 μg/mL FN-H89, or 10 μg/mL VCAM-1. Some cells were pretreated for 30 minutes with anti-α4 or -α5 mAbs (10 μg/mL) and then incubated in the presence of the Abs. After 24 hours, the conditioned media were concentrated 50 times and analyzed by gelatin zymography. Values for MMP-9 production (arbitrary units) represent the average of all 6 samples studied; basal levels of MMP-9 on BSA were normalized to 100. (B) B-CLL cells from one patient were incubated on wells coated with the indicated concentrations of FN-H89 or VCAM-1, and after 24 hours analyzed as explained. (C) B-CLL cells from 2 patients (5 × 105/100 μL) were incubated on the upper chamber of Transwell filters coated with resting or TNF-α–activated HUVECs, and in the presence or absence of the indicated mAbs. After 24 hours, the conditioned media were concentrated 5 times and analyzed by gelatin zymography. Values for MMP-9 production (arbitrary units) represent the average of the 2 samples studied. Error bars indicate standard deviation. * P < .05; ** P < .01.
We also studied whether MMP-9 was up-regulated by adhesion of B-CLL cells to HUVECs. Unstimulated or TNF-α–activated HUVECs secreted only minimal levels of MMP-9 (Figure 2C). Adhesion of B-CLL cells to unstimulated HUVECs, did not increase MMP-9 production above the basal levels of cells on BSA (Figure 2C). However, adhesion to TNF-α–activated HUVECs significantly increased MMP-9 secretion, and this was blocked by anti-α4 mAbs (Figure 2C). In contrast, blocking αLor β2 integrin subunits had a minor effect (Figure 2C). MMP-9 up-regulation was also inhibited by anti–VCAM-1 but not anti–ICAM-1 mAbs (Figure 2C), further supporting the role of α4β1-mediated interactions in up-regulating MMP-9 production.
α4β1 integrin up-regulates MMP-9 in B-CLL cells via activation of the PI3-K/Akt/NF-κB signaling pathway
To determine the mechanism involved in the α4β1-mediated up-regulation of MMP-9, B-CLL cells from 3 different patients were incubated on FN-H89 in the presence of various protein kinase inhibitors. Gelatin zymographic analysis of the conditioned media revealed that inhibition of PKC, p38 MAPK, Src-family kinases, or ERK1/2 signaling did not affect the enhanced MMP-9 secretion (Figure 3A). In contrast, inhibition of PI3-K by wortmannin reduced this secretion to basal levels (Figure 3A). Wortmannin (or the other inhibitors; not shown) had no effect on the MMP-9 basal production of B-CLL cells on BSA (Figure 3A). To confirm these results, we used 2 different doses of wortmannin and LY294002, another inhibitor of PI3-K; we also tested the effect of API-2, a specific inhibitor of the PI3-K effector Akt. As shown in Figure 3B for patient 4, wortmannin and LY294002 produced a dose-dependent inhibitory effect, and API-2 reduced MMP-9 production to basal levels. At the concentration used, none of the inhibitors induced B-CLL apoptosis, as confirmed by annexin V uptake (Figure S2).
Having established that up-regulation of MMP-9 by α4β1 integrin involved the PI3-K/Akt signaling pathway, we next studied whether α4β1-mediated cell adhesion activated Akt. B-CLL cells from 2 representative samples were incubated on FN-H89 or BSA. After 24 hours, cells were lysed and lysates analyzed by Western blotting. As shown in Figure 3C, B-CLL cell adhesion to FN-H89 (but not to BSA) induced Akt phosphorylation, and this was completely blocked by wortmannin or API-2. α4β1 integrin–mediated adhesion did not activate ERK1/2 (Figure 3C), indicating that the effect was specific for Akt. Moreover, the Akt downstream effector NF-κB was also activated at this time, as determined by measuring the levels of the NF-κB–associated protein IκB-α, which is released from the complex and degraded upon activation.29 As shown in Figure 3D, IκB-α decreased upon cell adhesion to FN-H89, and this response was reverted by PI3-K or Akt inhibitors. We also studied the kinetics of Akt phosphorylation induced by B-CLL adhesion to FN-H89. Figure 3E shows that Akt was phosphorylated after 30 minutes and remained activated for the 24 hours of the assay. Sustained Akt signaling was further confirmed by kinetic studies of IκB-α levels, which remained low throughout the assay (Figure S3). No ERK1/2 activation was observed at any time (Figure 3E).
α4β1 integrin–induced MMP-9 up-regulation involves the PI3-K/Akt signaling pathway. (A) B-CLL cells from 3 different patients were incubated or not with either 30 nM wortmannin (Wmn) or 5 μM of the following: Bis I, SB203580 (SB), PP2, and UO126 for 1 hour at 37°C, and added to FN-H89–coated wells. Cells untreated or treated with 30 nM Wmn were also added to BSA-coated wells. After 24 hours, the conditioned media were concentrated and analyzed by gelatin zymography, and MMP-9 secretion was quantitated. Basal levels of MMP-9 on BSA were normalized to 100, and values represent arbitrary units. (B) B-CLL cells from one patient were preincubated for 1 hour with the indicated concentrations of wortmannin, LY294002 (LY), or API-2, and added to FN-H89–coated wells. After 24 hours, the conditioned media were analyzed by gelatin zymography. Values represent the average of duplicate determinations. (C) B-CLL cells (2 × 106) from 2 patients were incubated on 0.5% BSA or 10 μg/mL FN-H89 in the absence or presence of wortmannin or API-2. Cells were lysed after 24 hours and phosphorylated, and total Akt and ERK were analyzed by Western blotting using specific Abs. (D) The same lysates used in panel C were also analyzed by Western blotting using specific Abs to IκB-α. Relative IκB-α levels were quantitated and values represent the average of the 2 samples studied. IκB-α levels of cells on BSA were normalized to 1. (E) B-CLL cells (2 × 106) from one patient were incubated on 0.5% BSA or 10 μg/mL FN-H89, and at the indicated times phospho-Akt and phospho-ERK were analyzed as explained. Relative P-Akt and P-ERK levels were quantitated and constitutive levels were normalized to 1. Error bars indicate standard deviation. * P < .05; ** P < .01.
α4β1 integrin–induced MMP-9 up-regulation involves the PI3-K/Akt signaling pathway. (A) B-CLL cells from 3 different patients were incubated or not with either 30 nM wortmannin (Wmn) or 5 μM of the following: Bis I, SB203580 (SB), PP2, and UO126 for 1 hour at 37°C, and added to FN-H89–coated wells. Cells untreated or treated with 30 nM Wmn were also added to BSA-coated wells. After 24 hours, the conditioned media were concentrated and analyzed by gelatin zymography, and MMP-9 secretion was quantitated. Basal levels of MMP-9 on BSA were normalized to 100, and values represent arbitrary units. (B) B-CLL cells from one patient were preincubated for 1 hour with the indicated concentrations of wortmannin, LY294002 (LY), or API-2, and added to FN-H89–coated wells. After 24 hours, the conditioned media were analyzed by gelatin zymography. Values represent the average of duplicate determinations. (C) B-CLL cells (2 × 106) from 2 patients were incubated on 0.5% BSA or 10 μg/mL FN-H89 in the absence or presence of wortmannin or API-2. Cells were lysed after 24 hours and phosphorylated, and total Akt and ERK were analyzed by Western blotting using specific Abs. (D) The same lysates used in panel C were also analyzed by Western blotting using specific Abs to IκB-α. Relative IκB-α levels were quantitated and values represent the average of the 2 samples studied. IκB-α levels of cells on BSA were normalized to 1. (E) B-CLL cells (2 × 106) from one patient were incubated on 0.5% BSA or 10 μg/mL FN-H89, and at the indicated times phospho-Akt and phospho-ERK were analyzed as explained. Relative P-Akt and P-ERK levels were quantitated and constitutive levels were normalized to 1. Error bars indicate standard deviation. * P < .05; ** P < .01.
CXCL12 up-regulates MMP-9 in B-CLL cells via the ERK1/2/c-Fos signaling pathway and independently of PI3-K
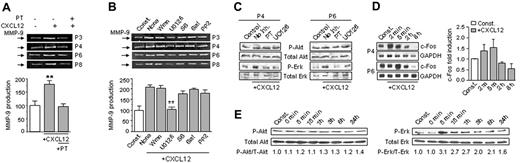
B-CLL cells express functional receptors for CXCL12 (CXCR4), which are involved in the migration of these cells particularly in the bone marrow.30,31 To establish whether the interaction CXCL12/CXCR4 could modulate MMP-9 production, B-CLL cells from 6 different patients were incubated for 24 hours with or without CXCL12 and the conditioned media analyzed by gelatin zymography. As shown in Figure 4A for 4 representative samples and quantitated for all 6 patients studied, CXCL12 significantly enhanced MMP-9 secretion with respect to untreated cells. This effect was completely blocked by inhibiting CXCR4 signaling with pertussis toxin (Figure 4A).
To determine whether this CXCL12 regulatory effect involved the same or different signaling pathways as α4β1 integrin, we performed the same experiments described in Figure 4A in the presence of various protein kinase inhibitors. As shown in Figure 4B for 4 representative samples, inhibition of ERK1/2 by UO126 significantly reduced MMP-9 to basal levels, while all other inhibitors, including wortmannin and LY294002 (not shown), had no effect. To determine whether CXCL12 induced ERK1/2 phosphorylation, B-CLL cells from the same 2 samples used in Figure 3C were incubated in the presence or absence of CXCL12. After 24 hours, cells were lysed and lysates analyzed by Western blotting. Figure 4C shows that CXCL12 phosphorylated ERK1/2 in B-CLL cells and this was inhibited by UO126 and pertussis toxin. CXCL12 did not activate Akt (Figure 4C). We next studied the activation of c-fos, a well-known effector of ERK1/2.32 c-fos was not detected after 24 hours of CXCL12 stimulation in the 2 samples studied (not shown), and thus we measured c-fos activation at earlier times. Figure 4D shows that CXCL12 rapidly increased (2-5 minutes) the basal expression of c-Fos, after which c-Fos levels progressively declined. In correlation with this peak of c-Fos activation, the levels of phospho-ERK1/2 were also maximal after 5 minutes of CXCL12 stimulation (Figure 4E). Although these levels also decreased with time, ERK1/2 remained activated for at least 24 hours, as previously observed (Figure 4C,E). CXCL12 did not activate Akt at any time (Figure 4E).
CXCL12 up-regulates MMP-9 in B-CLL cells via the ERK1/2 signaling pathway. (A) B-CLL cells (2 × 106) from 4 different patients were suspended in RPMI, 0.1% FCS and incubated on wells coated with 0.5% BSA and with or without 150 ng/mL CXCL12. Pertussis toxin (PT, 200 ng/mL) was added to some cells. After 24 hours, the conditioned media were concentrated and analyzed by gelatin zymography. Values represent the average of the 6 samples studied, and basal levels of MMP-9 without CXCL12 were normalized to 100. (B) B-CLL cells from 4 patients were incubated for 1 hour with or without (None) the indicated inhibitors (same concentrations as in Figure 3), and added to BSA-coated wells in the presence or absence of 150 ng/mL CXCL12. After 24 hours, the conditioned media were concentrated and MMP-9 was analyzed and quantitated. (C) Cells from 2 patients were incubated on 0.5% BSA in the presence of 150 ng/mL CXCL12, and in the presence or absence of pertussis toxin (PT) or UO126. Control cells were incubated in the absence of CXCL12. Phospho- and total Akt and ERK were analyzed by Western blotting. (D) B-CLL cells from the same patients used in panel C were incubated with or without 150 ng/mL CXCL12; at the indicated times, RNA was extracted and expression of c-fos mRNA was analyzed by RT-PCR using specific primers. GAPDH was also amplified as an internal control for sample loading. Values represent the average of the 2 samples studied. (E) Cells (2 × 106) from the same patient used in Figure 3E were incubated with or without 150 ng/mL CXCL12; at the indicated times, cells were lysed and lysates analyzed by Western blotting using specific Abs to phospho-Akt and phospho-ERK. Relative P-Akt and P-ERK levels were quantitated and normalized with respect to constitutive levels, which were considered 1. ** P < .01.
CXCL12 up-regulates MMP-9 in B-CLL cells via the ERK1/2 signaling pathway. (A) B-CLL cells (2 × 106) from 4 different patients were suspended in RPMI, 0.1% FCS and incubated on wells coated with 0.5% BSA and with or without 150 ng/mL CXCL12. Pertussis toxin (PT, 200 ng/mL) was added to some cells. After 24 hours, the conditioned media were concentrated and analyzed by gelatin zymography. Values represent the average of the 6 samples studied, and basal levels of MMP-9 without CXCL12 were normalized to 100. (B) B-CLL cells from 4 patients were incubated for 1 hour with or without (None) the indicated inhibitors (same concentrations as in Figure 3), and added to BSA-coated wells in the presence or absence of 150 ng/mL CXCL12. After 24 hours, the conditioned media were concentrated and MMP-9 was analyzed and quantitated. (C) Cells from 2 patients were incubated on 0.5% BSA in the presence of 150 ng/mL CXCL12, and in the presence or absence of pertussis toxin (PT) or UO126. Control cells were incubated in the absence of CXCL12. Phospho- and total Akt and ERK were analyzed by Western blotting. (D) B-CLL cells from the same patients used in panel C were incubated with or without 150 ng/mL CXCL12; at the indicated times, RNA was extracted and expression of c-fos mRNA was analyzed by RT-PCR using specific primers. GAPDH was also amplified as an internal control for sample loading. Values represent the average of the 2 samples studied. (E) Cells (2 × 106) from the same patient used in Figure 3E were incubated with or without 150 ng/mL CXCL12; at the indicated times, cells were lysed and lysates analyzed by Western blotting using specific Abs to phospho-Akt and phospho-ERK. Relative P-Akt and P-ERK levels were quantitated and normalized with respect to constitutive levels, which were considered 1. ** P < .01.
α4β1 integrin and CXCR4 independently up-regulate MMP-9
The preceding results indicated that CXCR4- and α4β1 integrin–dependent up-regulation of MMP-9 had different signaling requirements. We next studied whether the effect of both receptors was additive. B-CLL cells from 4 different patients were incubated for 24 hours on BSA or FN-H89 and with or without CXCL12, and the conditioned media were analyzed by gelatin zymography. Of interest, α4β1 integrin and CXCR4 costimulation did not increase MMP-9 secretion above the levels induced by either stimulus, but resembled the effect of CXCL12 alone (Figure 5A). To confirm this, we studied the signaling pathways involved in MMP-9 up-regulation by costimulation via α4β1 and CXCL12. As shown in Figure 5B for 2 representative samples, inhibition of ERK1/2 reduced the enhanced MMP-9 secretion by 80%, while inhibition of PI3-K resulted in 48% reduction. The combination of both inhibitors decreased MMP-9 to basal levels (94% inhibition, Figure 5B). All together, these results established that CXCL12 and α4β1 enhanced MMP-9 production via different signaling pathways and that the combination of both stimuli was not cooperative.
MMP-9 is involved in B-CLL invasion through basement membranes and transendothelial migration, in a PI3-K/Akt– and ERK1/2-dependent manner
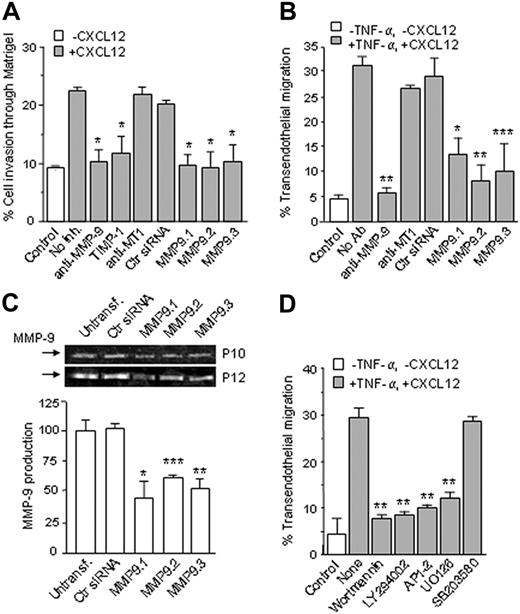
It was recently shown that MMP-9, when activated by plasmin, contributes to B-CLL migration through mixtures of collagen IV/laminin or HUVECs.18 We analyzed whether the enhanced MMP-9 production observed in the present study had a functional role in B-CLL invasion and transendothelial migration. For invasion assays we used Matrigel, which is mainly composed of laminin and type IV collagen and resembles vascular basement membranes. Although few cells migrated through Matrigel in the absence of chemokine, CXCL12 increased this number 2.5-fold (Figure 6A). Invasion through Matrigel was significantly inhibited by anti–MMP-9 Abs (86%) or the MMP-9 inhibitor TIMP-1 (80%), while an anti–MT1-MMP mAb had no effect (Figure 6A). These results were further confirmed by MMP-9 gene silencing experiments. B-CLL cells from 3 patients were transfected with 3 different siRNAs for MMP-9 or a control siRNA, and their invasion through Matrigel was measured. As shown in Figure 6A, all 3 MMP-9 siRNAs significantly reduced B-CLL invasion, while the control siRNA had no effect. Transfection with these siRNAs did not significantly reduced B-CLL viability with respect to controls (shown for one patient in Figure S4).
α4β1 integrin and CXCL12 independently enhance MMP-9 production in B-CLL cells. (A) B-CLL cells from 4 different patients were added to BSA- or FN-H89–coated wells and in the presence or absence of 150 ng/mL CXCL12. After 24 hours, MMP-9 secretion was analyzed by gelatin zymography and quantitated. Basal MMP-9 levels of cells on BSA and without CXCL12 were normalized to 100. (B) B-CLL cells from 2 patients were incubated on FN-H89 in the presence or absence (None) of the indicated kinase inhibitors. CXCL12 (150 ng/mL) was also added to the cells. Control cells (Contr) were incubated in 0.5% BSA and without CXCL12. The conditioned media were collected after 24 hours and MMP-9 was quantitated. Basal values were normalized to 100. * P < .05; ** P < .01.
α4β1 integrin and CXCL12 independently enhance MMP-9 production in B-CLL cells. (A) B-CLL cells from 4 different patients were added to BSA- or FN-H89–coated wells and in the presence or absence of 150 ng/mL CXCL12. After 24 hours, MMP-9 secretion was analyzed by gelatin zymography and quantitated. Basal MMP-9 levels of cells on BSA and without CXCL12 were normalized to 100. (B) B-CLL cells from 2 patients were incubated on FN-H89 in the presence or absence (None) of the indicated kinase inhibitors. CXCL12 (150 ng/mL) was also added to the cells. Control cells (Contr) were incubated in 0.5% BSA and without CXCL12. The conditioned media were collected after 24 hours and MMP-9 was quantitated. Basal values were normalized to 100. * P < .05; ** P < .01.
CXCL12-triggered transendothelial migration was also nearly completely inhibited (95%) by anti–MMP-9 but not anti–MT1-MMP Abs (Figure 6B). As in the case of Matrigel invasion, transfection of B-CLL cells with MMP-9 siRNAs significantly blocked transendothelial migration, while the control siRNA had no effect (Figure 6B). Blocking α4β1 integrin also inhibited B-CLL transendothelial migration (not shown), indicating that α4β1 is also involved in this process, as recently documented.7
To confirm that the siRNAs were functional, the conditioned medium of transfected B-CLL cells cultured for 24 hours was analyzed by gelatin zymography. As shown in Figure 6C for 2 representative samples, all 3 siRNAs significantly diminished MMP-9 secretion, while the control siRNA had no effect. HUVEC-enhanced MMP-9 production was also reduced by 66% upon transfection with MMP-9 siRNAs (not shown). All together, these results clearly established that MMP-9 plays a key role in B-CLL cell invasion across basement membranes and in transendothelial migration.
We also studied the signaling pathways required for B-CLL transendothelial migration. As shown in Figure 6D for 2 representative patients, inhibition of PI3-K/Akt or ERK1/2 activities blocked B-CLL cell transendothelial migration, while inhibition of p38 MAPK (used as control) had no effect. These results indicated that signals provided by α4β1 integrin (upon binding to VCAM-1 in endothelium) and CXCL12 were required for B-CLL cell transendothelial migration, and further supported the role of MMP-9, which is up-regulated in response to these signals, in this process.
Cell-associated MMP-9 localizes to the membrane as proactive and active forms, and to α4β1-induced podosomes, where it degrades extracellular matrix
MMP-9 is detected at the B-CLL cell surface by flow cytometry and is able to degrade collagen.18 We studied the localization of cellular MMP-9 and its possible modulation by α4β1 integrin. B-CLL cells were incubated on FN-H89 for 24 hours and, after lysing, the membrane and cytosolic fractions analyzed by gelatin zymography. Figure 7A shows that MMP-9 was located mainly at the cell membrane, and that the mature form of 85 kDa was present in this fraction. The mature form was also constitutively present at the cell membrane (Figure 7A), confirming that α4β1 integrin regulates MMP-9 by increasing production of the proform, rather than affecting MMP-9 activation at the cell surface.
It was previously shown that B-CLL cells, but not normal B cells, form podosomes,33,34 which are cytoskeletal structures in close contact with the substratum and associated with cell migration/ invasion.35 We then studied whether α4β1 engagement induced podosome formation and whether MMP-9 localized to these structures. Confocal microscopy analyses showed that B-CLL cells incubated on FN-H89 or VCAM-1, but not p-Lys, formed the punctuate pattern of an actin-rich core surrounded by a vinculin-containing ring characteristic of podosomes35 (Figure 7B). Approximately 15% to 25% of cells formed podosomes, depending on the sample (6 samples studied). CXCL12 did not induce podosomes and did not affect induction of podosomes by α4β1 integrin (not shown), confirming that integrin-mediated adhesion was a prerequisite for podosome formation. Of importance, α4β1 engagement induced MMP-9 colocalization with actin at the podosome core, as documented in Figure 7C using dot-plot analyses. Some MMP-9 also colocalized with cortical actin (Figure 7C-D).
Role of MMP-9 in B-CLL migration through Matrigel and HUVECs. (A) B-CLL cells (5 × 105) from 3 different patients, with or without previous incubation with the indicated Abs (10 μg/mL), TIMP-1 (1.5 nM), or transfected with control siRNA or 3 different MMP-9 siRNAs (MMP9.1, MMP9.2, and MMP9.3), were added to the upper chamber of Transwell filters coated with Matrigel. CXCL12 (150 ng/mL) was added to the medium in the bottom chamber, except for the control. After 24 hours, invasive cells were counted by flow cytometry. Values represent the average of the 3 samples studied and are expressed as the percentage of total cells added. (B) B-CLL cells from 3 patients, with or without previous incubation with the indicated Abs, or transfected with the same siRNAs shown in panel A, were added to Transwell filters previously coated with inactivated (control) or TNF-α–activated HUVECs. CXCL12 was added to the bottom chamber except for the control. After 24 hours, transmigrated cells in the bottom chamber were counted by flow cytometry. Values represent the average of the 3 samples studied. (C) Transfected B-CLL cells from 2 patients were incubated for 24 hours, and the conditioned medium was concentrated and analyzed by gelatin zymography. Values are the average of the 2 samples studied. (D) B-CLL cells (5 × 105) from 2 different patients were incubated for 1 hour with the indicated inhibitor or with medium (None) and added to Transwell filters coated with TNF-α–activated HUVECs. Control cells were added to inactivated HUVECs. After 24 hours, cells in the bottom chamber were counted by flow cytometry. Values represent the average of the 2 samples studied. * P < .05; ** P < .01; *** P < .001.
Role of MMP-9 in B-CLL migration through Matrigel and HUVECs. (A) B-CLL cells (5 × 105) from 3 different patients, with or without previous incubation with the indicated Abs (10 μg/mL), TIMP-1 (1.5 nM), or transfected with control siRNA or 3 different MMP-9 siRNAs (MMP9.1, MMP9.2, and MMP9.3), were added to the upper chamber of Transwell filters coated with Matrigel. CXCL12 (150 ng/mL) was added to the medium in the bottom chamber, except for the control. After 24 hours, invasive cells were counted by flow cytometry. Values represent the average of the 3 samples studied and are expressed as the percentage of total cells added. (B) B-CLL cells from 3 patients, with or without previous incubation with the indicated Abs, or transfected with the same siRNAs shown in panel A, were added to Transwell filters previously coated with inactivated (control) or TNF-α–activated HUVECs. CXCL12 was added to the bottom chamber except for the control. After 24 hours, transmigrated cells in the bottom chamber were counted by flow cytometry. Values represent the average of the 3 samples studied. (C) Transfected B-CLL cells from 2 patients were incubated for 24 hours, and the conditioned medium was concentrated and analyzed by gelatin zymography. Values are the average of the 2 samples studied. (D) B-CLL cells (5 × 105) from 2 different patients were incubated for 1 hour with the indicated inhibitor or with medium (None) and added to Transwell filters coated with TNF-α–activated HUVECs. Control cells were added to inactivated HUVECs. After 24 hours, cells in the bottom chamber were counted by flow cytometry. Values represent the average of the 2 samples studied. * P < .05; ** P < .01; *** P < .001.
Induction of podosomes by α4β1 integrin did not require PI3-K or ERK1/2 activity, as actin still presented a punctuate pattern in the presence of wortmannin or UO126 (Figure 7D left panels). However, inhibition of PI3-K completely prevented MMP-9 localization in podosomes, demonstrated by confocal dot-plot analyses (Figure 7D). In contrast, inhibition of ERK1/2 did not affect this MMP-9 localization (Figure 7D).
We next studied whether MMP-9 was active in podosomes by examining the degradation of a gelatin/fibronectin matrix. In initial experiments with unlabeled gelatin and fibronectin, we established that adhesion of B-CLL cells to this matrix was completely dependent on α4β1 integrin (not shown) and resulted in formation of podosomes where MMP-9 colocalized with actin (Figure 7E). Subsequently, B-CLL cells were incubated on FITC-labeled gelatin/fibronectin for 24 hours and degradation was visualized by the local loss of matrix immunofluorescence. Figure 7F shows that B-CLL cells containing podosomes, but not those lacking them, degraded the gelatin/fibronectin matrix. This effect was completely inhibited by TIMP-1 (Figure 7F) or anti–MMP-9 Abs (not shown), confirming that MMP-9 was active in podosomes and responsible for the observed matrix degradation. Moreover, inhibition of PI3-K activity also blocked matrix degradation, while inhibition of ERK1/2 had no effect (Figure 7F). All together, these results established that α4β1 integrin in B-CLL cells regulates not only MMP-9 production but also its localization and activity in podosomes, and both processes involve PI3-K signaling.
Discussion
In this report, we have studied the role and regulation of MMP-9 in B-CLL cells. We show for the first time that (1) MMP-9 is up-regulated in response to distinct signals elicited by α4β1 integrin or CXCR4 ligand engagement; (2) MMP-9 plays a major role in transendothelial migration and basement membrane invasion; and (3) MMP-9 localizes to podosomes in an α4β1-dependent manner and degrades extracellular matrix.
Previous studies have shown that MMP-9 is constitutively present in lysates and culture medium of B-CLL cells.17,18 We have confirmed and extended these results by showing that MMP-9 expression in B-CLL cells was observed in all samples studied and was significantly higher than in normal B cells. Our study also establishes that MMP-9 is the major MMP in B-CLL, since, unlike other B-cell malignancies,36,37 MMP-2 was absent, confirming a previous report18 and MT1-MMP was not detected at the B-CLL cell surface even after stimulation.
Increasing evidence indicates that α4β1 integrin plays an important role in B-CLL progression. Elevated expression of α4β1 correlates with the presence of lymphadenopathy,5 and α4β1 is required, together with VEGF, for chemokine-directed B-CLL transendothelial migration.5,7 Besides its role in cell migration, we have previously reported that α4β1 protects B-CLL cells from spontaneous and drug-induced apoptosis.28,38 Moreover, gene and surface-antigen expression profiling have recently established that the correlated overexpression of α4 integrin subunit and CD38 is characteristic of bad B-CLL prognosis.39,40 We now show in the present study that α4β1 ligand engagement up-regulates MMP-9 production and induces podosomes and the localization of active MMP-9 in these structures, thus affecting the migratory and invasive properties of B-CLL cells.
Our results also establish that PI3-K/Akt are the kinases involved in α4β1-induced up-regulation of MMP-9. Previous reports have shown that the PI3-K/Akt/NF-κB pathway is constitutively activated in fresh B-CLL cells and that Akt phosphorylation decreases upon cell culture.41 We show here that α4β1/ligand engagement increases the constitutive levels of phospho-Akt and induces sustained Akt/NF-κB activation. Ringshausen et al42 have shown that active p38 MAPK is required for spontaneous secretion of MMP-9 by B-CLL cells. We did not observe this requirement for the basal secretion of MMP-9 by cells on BSA, and this may be due to the different experimental conditions used in both studies. Of interest, up-regulation of MMP-9 by fibronectin in T cells was increased by inhibiting PI3-K and the MAPKs MEK1/2 and p38, and was dependent on Src activity.25 These observations suggest that MMP-9 regulation, even when triggered by cell adhesion via integrins, involves different signaling pathways depending of the cell type. In support of these differences, we found that both ligands of α4β1, FN-H89 and VCAM-1, up-regulated MMP-9, while in T cells VCAM-1 had no effect.26
Analysis of cell-associated MMP-9 in B-CLL cells. (A) B-CLL cells (3 × 106) from a representative sample were incubated on FN-H89 for 24 hours. Cells were removed and membrane and cytosolic fractions separated and subjected to gelatin zymography. Identical aliquots from the same lysate were analyzed by Western blotting using anti-RhoGDI–specific Abs. (B) B-CLL cells in medium containing 80 nM PMA were added to glass coverslips coated with 5 μg/mL p-Lys, 10 μg/mL FN-H89, or 10 μg/mL VCAM-1. After 1 hour at 37°C, podosomes were analyzed by confocal microscopy after staining F-actin (red) with Alexa 568–phalloidin and vinculin (green) with specific primary Abs/FITC-labeled secondary Abs. Inserts are ×20 magnifications. Bar represents 4 μm. (C) B-CLL cells were added to FN-H89– or VCAM-coated glass coverslips, and after 1 hour MMP-9 (green) was visualized with specific primary Abs and Alexa 488–labeled secondary Abs. F-actin (red) was stained as explained in panel B, and the merged images are shown. Colocalization (yellow) of MMP-9 and F-actin in podosomes was further demonstrated using dot-plot analyses as explained in “Materials and methods.” (D) B-CLL cells were preincubated or not for 1 hour with 30 nM wortmannin (Wmn) or 10 μM UO126 and added to glass coverslips coated with 10 μg/mL FN-H89. F-actin and MMP-9 were visualized as explained, and the merged images (yellow) and dot-plot analyses are shown. (E) B-CLL cells were added to coverslips coated with gelatin/fibronectin; after 24 hours, colocalization of actin and MMP-9 in podosomes was analyzed as explained. (F) B-CLL cells, with or without the indicated inhibitors, were added to coverslips coated with FITC-gelatin/fibronectin; after 24 hours, F-actin was stained with Alexa 568–phalloidin and sites of matrix degradation were visualized by the loss of green fluorescence. Arrows indicate cells containing podosomes. Bar represents 12 μm. Images were acquired using a confocal scanning inverted AOBS/SP2 microscope (Leica Microsystems, Heidelberg, Germany) with a 63×/1.3 NA PL-APO glycerol immersion objective. Leica's LCS 15.37 dye-separation software was used for colocalization studies; when necessary, Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) was used for image processing.
Analysis of cell-associated MMP-9 in B-CLL cells. (A) B-CLL cells (3 × 106) from a representative sample were incubated on FN-H89 for 24 hours. Cells were removed and membrane and cytosolic fractions separated and subjected to gelatin zymography. Identical aliquots from the same lysate were analyzed by Western blotting using anti-RhoGDI–specific Abs. (B) B-CLL cells in medium containing 80 nM PMA were added to glass coverslips coated with 5 μg/mL p-Lys, 10 μg/mL FN-H89, or 10 μg/mL VCAM-1. After 1 hour at 37°C, podosomes were analyzed by confocal microscopy after staining F-actin (red) with Alexa 568–phalloidin and vinculin (green) with specific primary Abs/FITC-labeled secondary Abs. Inserts are ×20 magnifications. Bar represents 4 μm. (C) B-CLL cells were added to FN-H89– or VCAM-coated glass coverslips, and after 1 hour MMP-9 (green) was visualized with specific primary Abs and Alexa 488–labeled secondary Abs. F-actin (red) was stained as explained in panel B, and the merged images are shown. Colocalization (yellow) of MMP-9 and F-actin in podosomes was further demonstrated using dot-plot analyses as explained in “Materials and methods.” (D) B-CLL cells were preincubated or not for 1 hour with 30 nM wortmannin (Wmn) or 10 μM UO126 and added to glass coverslips coated with 10 μg/mL FN-H89. F-actin and MMP-9 were visualized as explained, and the merged images (yellow) and dot-plot analyses are shown. (E) B-CLL cells were added to coverslips coated with gelatin/fibronectin; after 24 hours, colocalization of actin and MMP-9 in podosomes was analyzed as explained. (F) B-CLL cells, with or without the indicated inhibitors, were added to coverslips coated with FITC-gelatin/fibronectin; after 24 hours, F-actin was stained with Alexa 568–phalloidin and sites of matrix degradation were visualized by the loss of green fluorescence. Arrows indicate cells containing podosomes. Bar represents 12 μm. Images were acquired using a confocal scanning inverted AOBS/SP2 microscope (Leica Microsystems, Heidelberg, Germany) with a 63×/1.3 NA PL-APO glycerol immersion objective. Leica's LCS 15.37 dye-separation software was used for colocalization studies; when necessary, Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA) was used for image processing.
It is well known that CXCL12 enhances MMP-9 expression in myeloma, osteoclasts, and other cell types.37,43,44 Our present results constitute the first evidence that CXCL12 up-regulates MMP-9 in B-CLL cells. Of interest, CXCL12 up-regulation of MMP-9 was independent of the effect of α4β1 integrin and involved ERK1/2 but not Akt activation. ERK1/2 activation by CXCL12 has been previously observed in several cell systems,45,46 but we further show that in B-CLL cells, ERK1/2 phosphorylation was sustained and produced rapid c-fos activation. MMP-9 gene promoter contains 1 NF-κB and 2 API-1 (the complex of Fos and Jun) elements,9 and thus, although this study focused on MMP-9 regulation at the protein level, our results strongly suggest that α4β1 integrin and CXCL12 may transcriptionally regulate MMP-9. Nevertheless, modulation of MMP-9 at other levels cannot be disregarded. On the other hand, independent regulation of MMP-9 by α4β1 integrin and CXCL12 may provide the basis for a continuous stimulation of MMP-9 in physiological situations where only α4β1 ligands or chemokine is present.
Our results provide strong evidence for a functionally active MMP-9 in B-CLL cells. First, mature MMP-9 was present at the cell membrane. Second, anti–MMP-9 Abs, TIMP-1, or MMP-9 siRNAs significantly inhibited B-CLL invasion through basement membranes and transendothelial migration. Third, MMP-9 localized to α4β1-induced podosomes and efficiently degraded extracellular matrix. The fact that matrix degradation was also completely blocked by TIMP-1 or anti–MMP-9 Abs further supports a major role for MMP-9 in B-CLL invasion. MMP-9 has been found in podosomes in other cell types,35 but its localization in B-CLL cell podosomes specifically required PI3-K signaling. This requirement emphasizes the role of α4β1 integrin (which activates PI3-K/Akt) not only in up-regulating MMP-9 secretion but also in focalizing its activity in podosomes. This property appears to be unique for α4β1 since CXCL12, which also up-regulates MMP-9 secretion, did not induce podosomes.
Although these functional assays clearly established that MMP-9 is active in B-CLL cells, in conditioned media we did not observe the mature 85-kDa form. Many previous studies on different cell types have reported similar observations,47 and the reason for this remains unclear. One possibility is that soluble mature MMP-9 is produced at undetectable levels, yet sufficient for focalized activity. MMP-9 could also become active by alternative, nonproteolytic mechanisms involving oxidative modifications or conformational changes.47 It is not known whether these mechanisms operate in B-CLL cells. On the other hand, proteolytic activation of pro–MMP-9 is accomplished by a variety of proteases, including plasmin, and is thought to be more efficient at the cell surface than in solution.47,48 Indeed, we have observed active MMP-9 in B-CLL cell membranes and podosomes, suggesting that a fraction of secreted MMP-9 binds to the cell surface. MMP-9 can associate with several “docking” molecules (CD44, Ku protein, ICAM-1, integrins) at the cell surface, thereby facilitating its activation and proteolytic activity.47-49 In this context, enhanced release of the proform by α4β1 integrin or CXCL12 in B-CLL cells may provide a continuous pool of zymogen, susceptible of activation as needed.
The present report therefore identifies novel physiological mechanisms contributing to B-CLL progression and provides evidence for a key role of MMP-9 in this malignancy. MMP-9 degrades extracellular matrix and mediates B-CLL migration and invasion, as we show here, but may also release matrix-bound angiogenic factors, such as VEGF and bFGF, and contribute to angiogenesis.11 Although still not proved in B-CLL, these angiogenic factors in turn up-regulate MMP-9 in other cell systems,11 thus establishing a feedback mechanism for tumor expansion. It is interesting that VEGF50,51 and the 2 molecules, α4β1 integrin28,38 and CXCL12,52 identified in the present report as physiological regulators of MMP-9 production, provide survival signals in B-CLL, and MMP-9 may also be part of this survival mechanism.42 Interferons can suppress MMP-9 production,17 and it was recently shown that hyperforin induces apoptosis of B-CLL cells by inhibiting the secretion of MMP-9 and VEGF,53 suggesting that MMP-9 could be a target for therapeutic intervention in B-CLL. Therapies aimed to neutralize several MMPs are already in progress for other pathologies.10,11 Since we have also identified the kinase signaling pathways involved in MMP-9 up-regulation, targeting these pathways, alone or in combination with other protocols, may also prove useful for B-CLL treatment.
Prepublished online as Blood First Edition Paper, July 13, 2006; DOI 10.1182/blood-2006-03-007294.
Supported by grant SAF2003-00824 from the Ministerio de Educación y Ciencia (MEC) and by the Fundación de Investigación Médica Mutua Madrileña (FMM). J.R.M. was supported by a fellowship from FMM; E.E.-D. was supported by a fellowship from MEC.
J.R.-M. performed research and designed some experiments; E.E.-D. performed research; R.S. performed the confocal microscopy analyses; M.J.T. contributed with patient samples and data; J.A.G.-M. contributed patient samples and data; and A.G.-P. designed and supervised research and wrote the paper.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the B-CLL patients who donated samples for this research; all mentioned investigators for antibodies or reagents; Drs Joaquín Teixidó and Alicia G. Arroyo for critical reading of the paper; Dr Paloma Sánchez-Mateos (Hospital Gregorio Marañón, Madrid) for valuable advice with confocal microscopy analyses; Dr Pedro Lastres for help with flow cytometry; and Mercedes Hernández del Cerro for excellent technical assistance.