There has been much discussion within the literature concerning the measurement of NO-related species that are formed in reaction with hemoglobin (Hb). These studies are of great interest in terms of understanding the role of Hb in relation to vascular control. However, these studies are inherently complex; the design and interpretation of such experiments must not be oversimplified. Problems inherent in such work were highlighted by a recent publication in Blood where oversimplification of the issues concerning NO and Hb interaction led to inappropriate conclusion.
In a plenary paper in Blood on the formation of S-nitrosohemoglobin (SNO-Hb), Huang et al broadly claimed that “... allosterically controlled intramolecular transfer from heme to thiol does not occur under normal conditions and therefore a defect in this processing of NO by Hb does not contribute to pathologic states.”1(p2604) Their reported work, however, does not support this claim.
Factors important to the effective transfer of the NO moiety from heme to thiol in Hb have recently been reviewed2 and they include the following: (1) the ratio of NO to heme and the absolute amount of NO added; (2) the manner of NO addition; and (3) the time of incubation of NO and Hb prior to oxygenation and the rate of oxygenation. These factors point to the importance of specific micropopulations of Hb2 in the chemistry of SNO formation. The reactive species responsible for S-nitrosylation are not fully elucidated; likewise, a general model that rationalizes the dependence of SNO formation on these factors has yet to be advanced. Accordingly, great caution is required in comparing experiments done under different conditions and in drawing general conclusions from experiments performed under some specific set of conditions. This caution is lacking in the article by Huang et al.1
The authors hypothesized that an 80% SNO-Hb yield would be obtained from oxygenation of Hb nitrosylated with an NO/heme ratio of 1:100. This prediction, however, is drawn from (arbitrarily selected) prior work carried out at Hb concentrations of 100 to 200 μM.3 (More recent work with similar results at 200 μM was ignored.4 ) In their studies, a substantially higher Hb concentration (> mM) was employed, even though such high concentrations had already been reported to pose technical problems; specifically, such viscous solutions are difficult to oxygenate rapidly and give reduced SNO-Hb yields. The more dilute Hb solutions, similar to Hb in red blood cells (RBCs) traversing the lung, are oxygenated in seconds, as opposed to the approximately 5 minutes needed by the authors to oxygenate their concentrated solutions. This delay allows well-known NO migration and auto-oxidation processes that reduce the efficiency of SNO formation. Thus, the experimental design of Huang et al is flawed. The authors neglect the importance of outpacing competing processes that disarm SNO-Hb production and make unjustified comparisons of results obtained at widely different NO and/or Hb levels. The results of Huang et al are insufficient to contradict findings of others obtained under substantially different conditions, conditions that are arguably more pertinent to the physiologic situation; their general conclusion cannot be validly inferred from the reported work.
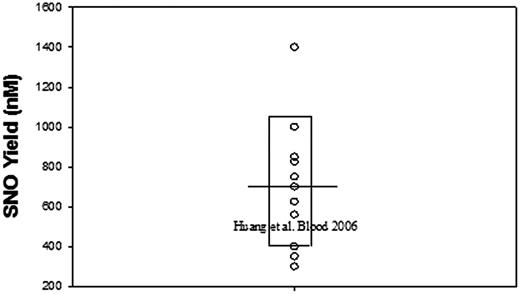
Finally, it should be noted that Huang et al1 actually do form amounts of SNO-Hb that are very similar to those obtained by others at comparable NO levels (∼20% of ∼10 μM added NO)3-6 (Figure 1). Interestingly, they report that the SNO-Hb is produced largely prior to oxygenation. This remarkable result raises questions about the composition of their samples. Moreover, given the equilibrium-like regulation of SNO formation, the large SNO-Hb cohort present before oxygenation could also be expected to limit oxygenation-induced SNO-Hb formation.
Exemplary literature values3-7for SNO yield upon oxygenation of Hb incubated with NO. Values are given irrespective of heme or NO level. The bar represents the mean and the box surrounds the single standard deviation limit.